Abstract
Recent studies have shown that certain copy number variations (CNV) are associated with a wide range of neurodevelopmental disorders, including autism spectrum disorders (ASD), bipolar disorder and intellectual disabilities. Implicated regions and genes have comprised a variety of post synaptic complex proteins and neurotransmitter receptors, including gamma-amino butyric acid A (GABAA). Clusters of GABAA receptor subunit genes are found on chromosomes 4p12, 5q34, 6q15 and 15q11-13. Maternally inherited 15q11-13 duplications among individuals with neurodevelopmental disorders are well described, but few case reports exist for the other regions. We describe a family with a 2.42 Mb duplication at chromosome 4p13 to 4p12, identified in the index case and other family members by oligonucleotide array comparative genomic hybridization, that contains 13 genes including a cluster of four GABAA receptor subunit genes. Fluorescent in-situ hybridization was used to confirm the duplication. The duplication segregates with a variety of neurodevelopmental disorders in this family, including ASD (index case), developmental delay, dyspraxia and ADHD (brother), global developmental delays (brother), learning disabilities (mother) and bipolar disorder (maternal grandmother). In addition, we identified and describe another individual unrelated to this family, with a similar duplication, who was diagnosed with ASD, ADHD and borderline intellectual disability. The 4p13 to 4p12 duplication appears to confer a susceptibility to a variety of neurodevelopmental disorders in these two families. We hypothesize that the duplication acts through a dosage effect of GABAA receptor subunit genes, adding evidence for alterations in the GABAergic system in the etiology of neurodevelopmental disorders.
Keywords: autism, bipolar disorder, chromosome disorders, DNA copy number variation, intellectual disabilities, GABAA
INTRODUCTION
Neurodevelopmental disorders encompass a wide variety of phenotypes that may include autism spectrum disorder (ASD), developmental delay (DD), intellectual disability (ID), seizures, attention deficit/hyperactivity disorder (ADHD), bipolar disorder and schizophrenia, among others. Early research in this area focused on defining very narrow phenotypes on which to perform genetic studies. It has emerged through copy number variantion (CNV) investigations that these disorders have considerable genetic overlap, indicating specific genetic defects may lead to a variety of phenotypes.1 As examples, separate studies based on the individual phenotypes of autism, intellectual disability, schizophrenia and bipolar disorder have shown that the recurrent CNVs 1q21 duplication, 15q11-q13 duplication or deletion, 16p11.2 duplication and 22q11.2 deletion are highly enriched among individuals with any one of these neurodevelopmental disorders compared with normal controls.2 While these CNVs and others individually are rare, collectively they account for 10–15% of all cases of DD/ID and ASD.3
Some of the genes encompassed by the CNVs observed in neurodevelopmental disorders encode proteins involved in the ubiquitin degradation pathway (UBE3A – 15q11), formation and maintenance of dendritic spines (SHANK3 – 22q13), the post synaptic density (NRXN1 – 2p16.3) and neurotransmitters such as gamma-amino butyric acid A (GABAA) receptors (GABRB3 – 15q12). The genes encoding subunits of the GABAA receptors demonstrate a clustering in their genomic arrangement, with four of these receptors located on chromosome 4p12 (GABRG1, GABRA2, GABRA4 and GABRB1), five on 5q34-35.1 (GABRB2, GABRA6, GABRA1, GABRG2 and GABRP), two on 6q15 (GABRR1 and GABRR) and three on 15q12 (GABRA5, GABRB3 and GABRG3). This might suggest that besides the cluster on 15q12 the other clusters may be involved in these disorders as well. However, while recurrent CNVs of 15q11-q13 are well known to cause neurodevelopmental disorders,4 reports of CNVs in the other regions among individuals with these problems are rare.
Here, we describe a family with a 2.42 Mb duplication spanning chromosome 4p13 to 4p12, that contains 13 genes including the cluster of GABAA receptor genes. Oligonucleotide microarray and fluorescence in-situ hybridization were used to identify this CNV in three generations, demonstrating segregation with a variety of neurodevelopmental phenotypes. In addition, we identified another individual with ASD unrelated to this family, with a similar duplication.
METHODS
The families were identified by retrospective review for reporting. The Institutional Review Board of Nationwide Children's Hospital approved of this case study. Details of their medical history and genetic testing were extracted from the medical records.
Array comparative genomic hybridization (aCGH) diagnostic testing was performed in the Clinical Cytogenetics and Molecular Genetics Laboratory at Nationwide Children's Hospital, Columbus, OH. The Family A index case was tested with SignatureSelect OS 105 k v1.1 oligonucleotide microarray (Agilent, Santa Clara, CA, USA). All other Family A members and individual 1 of Family B were tested with NimbleGen CGX-3 135 k oligonucleotide microarray (Roche NimbleGen, Madison, WI, USA). Confirmation of the duplication was performed by standard fluorescence in situ hybridization (FISH) analysis using a BAC clone specific to 4p12, either RP11-383M19 overlapping the GABRB1 gene or RP11-564P3 overlapping the GABRG1 gene (Signature Genomic Laboratories, Spokane, WA, USA) and a control probe CEP 4 (Abbott Molecular, Des Plaines, IL, USA) localizing at the centromere of chromosome 4.
Case report
Detailed case descriptions are available in the Supplementary Materials. A summary of the clinical information is presented here.
Family A
Individual IV-2
The index case is a 6-year-old Caucasian male (Figure 1). He was born to non-consanguineous parents at term with a birth weight of 3.96 kg. There were no known exposures to teratogenic agents. His early milestones were normal. He sat at 6 months of age, crawled at 12 months of age and walked by 18 months of age. He had his first words between 12 and 18 months of age, and soon after started speaking in short phrases. However, at 24 months of age he lost his language and communicative skills, and developed repetitive, stereotypic behaviors. At 2 years and 9 months of age the initial psychological evaluation was performed. He had a total score of 18 on Module 1 of the Autism Diagnostic Observation Schedule (ADOS), where the cutoff score for autism is 12 and for ASD is 7. Testing of intellectual skills included the Leiter-R (full scale IQ of 48) and the Stanford-Binet (abbreviated IQ score of 55). He was diagnosed with Autistic Disorder and Severe Mixed Receptive/Expressive Communication Disorder. Shortly after, he was seen in our Genetics Clinic for further evaluation of his autism and abnormal aCGH testing with 4p13 to 4p12 duplication. He had normal growth parameters (weight, height and head circumference) and normal physical examination. His previous hearing tests revealed adequate hearing to support normal speech and language development.
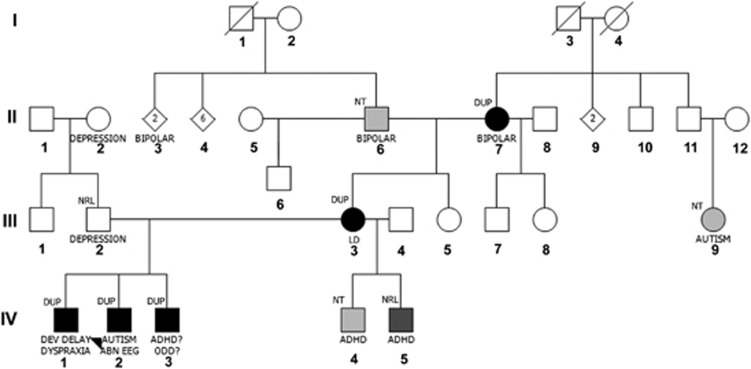
Figure 1.
Pedigree of Family A. Roman numerals indicate generation and Arabic numbers individuals in each generation. Filled symbols indicate affected individual; light gray indicates tested for duplication and not present, medium gray indicates not tested and black indicates tested and individual has duplication. Numbers inside symbols indicate number of individuals. DUP, duplication; NT, not tested; NRL, normal; ABN EEG, abnormal EEG; ADHD, attention deficit hyperactivity disorder; DEV DELAY, developmental delay; ODD, oppositional defiant disorder.
Additional diagnostic evaluations included magnetic resonance imaging (MRI) of the brain, fragile X testing, plasma amino acids, urine organic acids and creatine/guanidinoacetate (serum and urine) all of which were normal. Electroencephalogram (EEG) at age 44 months and 5 years was non-specifically abnormal, but no clinical seizure events have occurred.
Individual IV-1
The older brother of the index case first came to attention at age 2 years due to speech articulation problems. He has been diagnosed with fine motor delays and tremor, ADHD and sleep disorder (difficulty falling asleep with frequent awakening). Investigations at age 5 years included EEG, sleep studies and MRI. EEG showed abnormal sharp discharges without overt clinical seizures. Currently, he is in the 1st grade with an individual educational plan receiving speech, occupational and physical therapies. He takes methylphenidate for his ADHD. aCGH testing revealed he has the duplication.
Individual IV-3
The younger brother of the index case (currently 3 years old) has global developmental delays. He had his initial psychological evaluation at age 27 months. His ADOS Module 1 overall total score was 3, thus he did not meet criteria for an ASD. The Bayley III composite scales were normal, but expressive communication scale was low average. The development and behavior testing showed communication deficits, attention problems and aggressive behavior problems. The summary of his psychological evaluation revealed that he is at risk for ADHD and ODD. He receives speech therapy and has made significant improvements in both receptive and expressive language skills. He also has the duplication.
Additional affected/not affected/not tested individuals
The father of the index case (III-2) is 29-years-old and has depression; his aCGH testing was normal. He has a college degree. The mother of the three boys (III-3) is currently 36-years-old. She had anxiety and learning problems, specifically with mathematics, at school. She did not have any medical treatment or any services at school, and graduated from high school. Currently, she is taking college classes to become a special education teacher and continues having some difficulties with learning and anxiety. She does not have any medical diagnosis and takes no medication. aCGH testing showed that she carries the duplication. She has two additional boys aged 15 and 19 years from a previous partner. Both carry a clinical diagnosis of ADHD; the younger one (IV-5) had aCGH testing and did not carry the duplication, while the older one (IV-4) declined genetic testing. The maternal grandfather (II-6) and grandmother (II-7) both have a diagnosis of bipolar disorder. Both have had aCGH testing, and the grandmother has the duplication. The maternal grandmother's first cousin once removed with autism (III-9) has not been tested.
Family B
Individual 1
This 8-year-old boy was recently seen in our Genetics Clinic for evaluation of autism and abnormal aCGH testing, which revealed 4p13 to 4p12 duplication. He was born at term. His birth weight was 4.19 kg. No complications with the pregnancy, birth or during post-natal stay were reported. Milestones during infancy were normal. He used about 20 words when he was aged 18 months; however, he suddenly stopped using words, and then began using them again at around 36 months. He was diagnosed with speech delay and possible autism at his local school district, and started preschool services between 2 ½–3 years. He also started private speech therapy at age 3 years. With therapies, he slowly gained words back. Formal psychological evaluations were performed at age 7 years and 7 months. His ADOS Module 2 score was 24, with cutoff for autism at 8 and ASD at 9. His family history includes ADHD and dyslexia in the father, and muscular dystrophy (specific type unknown) in the father, paternal uncle and paternal grandfather. The mother is healthy; there are several maternal relatives with possible bipolar disorder. A 3-year-old brother is reportedly healthy. There is no family history of ASD or ID.
RESULTS
The duplication within chromosome bands 4p13 to 4p12 (Figure 2) in Family A is 2.42 Mb in size (genomic coordinates UCSC Genome Browser Build hg18 Mar 2006: 45,542,188-47,968,330) and contains 13 genes including a cluster of four GABAA receptor subunit genes (GABRG1, GABRA2, GABRA4, GABRB1) and nine other genes – COX7B2, ATP10D (mitochondrial proteins), COMMD8 (COMM domain containing 8), NFXL1, (zinc-finger protein), NIPAL1, CORIN (pro-ANP to ANP cleavage), CNGA1 (cyclic nucleotide gated channel in retinal rod cells), TXK (tyrosine kinase regulating T-cell proliferation), TEC (tyrosine kinase important in osteoclastogenesis). FISH testing (Figure 3) confirmed this duplication showing three signals using a BAC probe within 4p12 GABRG1 gene. The duplication in individual 1 of Family B spanned the same region at 4p13p12 of the same size of 2.42 Mb (genomic coordinates 45,544,763-47,968,280). FISH analysis with RP11-383M19 confirmed the duplication in 92% of the interphase cells analysed. Metaphase-spread FISH showed two signals of the probe. The father of individual 1 in Family B has been tested and does not have the duplication; the mother has declined testing at this time. The diagnostic lab conducting array CGH for these individuals has performed a total of 6185 analyses (January 2009 to December 2012), and has not identified any other individuals with this duplication. Thus the incidence of the 4p13 to 4p12 duplication in this tested population is 2/6185 (frequency=0.0003).
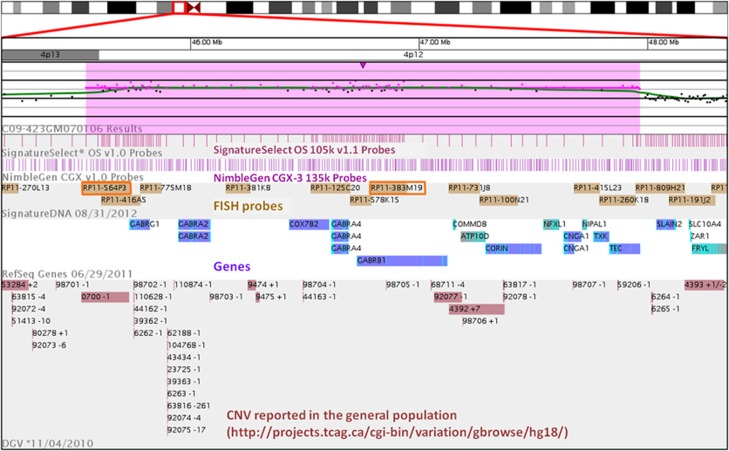
Figure 2.
Screen shot of aCGH on index case for chromosome 4:45081221-48453558. From top to bottom, ideogram of chromosome 4, log (R) intensity plot by oligonucleotide probe, oligonucleotide probe coverage for each array platform, location of BAC probes, RefSeq genes and CNVs from the Database of Genomic Variation.18 Duplicated region is highlighted in pink. OMIM genes in region (9 total) include GABRG1, GABRA2, COX7B2, GABRA4, GABRB1, CORIN, CNGA1, TXK, TEC; other genes in region (4 total) are COMMD8, ATP10D, NFXL1, NIPAL1. Red boxes indicate BAC probes used for FISH studies.
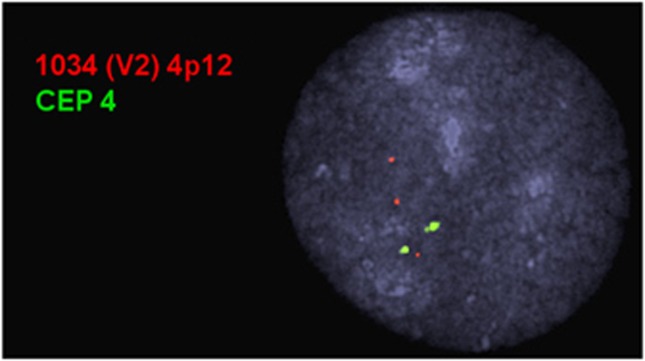
Figure 3.
Fluorescent in-situ hybridization for 4p13 to 4p12 duplication. FISH analysis on the index case using BAC clone RP11-564P3 (red) demonstrated three signals for 4p12 in 70% (28/40) of interphase cells counted, suggestive of a duplication at 4p12. A simultaneously hybridized control probe CEP 4 (green) showed a normal signal and pattern. Probe genomic location noted in Figure 2.
DISCUSSION
We report a family with a 2.42 Mb duplication within chromosome band 4p13 to 4p12 that tracks through three generations. The affected family members present with different neurodevelopmental phenotypes, which include developmental, motor and speech delays, learning disabilities, autism and bipolar disorder. In addition, we describe an unrelated individual with autism and ADHD possessing the same duplication.
Few cases have been published regarding alterations of chromosome 4p12 in autistic individuals that include the GABAA receptor subunit gene cluster. One case report describes a 3-year-old girl with autism and mosaicism for duplication of 4p12 to 4p16 (mos 46,XX,dup(4)(p12p16)[54]/46,XX[6]) containing GABRG1, GABRA2 and GABRA4 receptor subunit genes. This chromosomal abnormality was found to be a de novo alteration because both her parents were healthy and have normal chromosomes.5 Another case report describes two brothers, 12 and 11-years-old, with autism and a maternally inherited paracentric inversion of a short arm of chromosome 4 [46,XY,inv(4)(p12p15.3)mat] disrupting the GABRG1 receptor gene, which was inherited from their mother.6 Last, there is one case report of an 18-year-old female with autistic disorder, intellectual disability and a duplication of 4p13 to 4p12 [46,XX,dup(4)(p12p13)]. Her mother has a history of major depressive disorder, but it was not reported if she also had the same chromosome abnormality.7
A query of the International Standards for Cytogenomic Arrays Consortium database (https://www.iscaconsortium.org/) on 21 November, 2012 identified 6 reports of gains and 1 report of a loss in this region, none of which precisely matches the 2.42 Mb gain we describe here (Table 1). Of note, all the phenotypes attributed to the CNVs are neurodevelopmental disorders. A similar search of the Database of Genomic Variants of control individuals (http://dgvbeta.tcag.ca/dgv/app/home) is shown in Figure 2, and shows only small infrequent CNVs.
Table 1. Case reports obtained from the International Standards for Cytogenomic Arrays Consortium array CGH database demonstrating an abnormality involving 4p13 to 4p12.
| Variant ID | Location (NCBI36/hg18) | Size (kb) | Type | Phenotypes | Inheritance | Interpretation |
|---|---|---|---|---|---|---|
| nssv578973 | Chr4:44052975-62794195 | 18 753 | Gain | DD plus | De novo | Pathogenic |
| nssv578974 | Chr4:44274452-48778047 | 4503 | Gain | Autism, DD, seizures | Not reported | Pathogenic |
| nssv582611 | Chr4:45389432-45755340 | 365 | Loss | ADHD | Not reported | Uncertain |
| nssv584481 | Chr4:40193250-49276624 | 9083 | Gain | DD plus | De novo | Pathogenic |
| nssv1415048 | Chr4:26424-47188369 | 47 457 | Gain | DD plus | Not reported | Pathogenic |
| nssv1495262 | Chr4:39122601-45813920 | 6672 | Gain | DD plus | Not reported | Uncertain |
| nssv1601963 | Chr4:47079898-48548634 | 1468 | Gain | DD plus | Not reported | Uncertain |
Abbreviations: ADHD, attention deficit hyperactivity; DD, developmental delay; DD plus, Developmental delay and additional significant developmental and morphological phenotypes referred for genetic testing.
We hypothesize the 4p13 to 4p12 duplication acts by a dosage effect of the GABAA receptor subunit gene cluster. GABAergic interneurons comprise only 10% of all brain neurons, but have vital roles by inhibitory modulation of neuronal excitability. GABA also is important in embryonic development, regulating a variety of developmental mechanisms.8 The other duplicated genes in the region, COX7B2, ATP10D (mitochondrial proteins), COMMD8 (COMM domain containing 8), NFXL1 (zinc-finger protein), NIPAL1, CORIN (pro-ANP to ANP cleavage) CNG1 (cyclic nucleotide gated channel in retinal rod cells), TXK (tyrosine kinase regulating T-cell proliferation), TEC (tyrosine kinase important in osteoclastogenesis), are not involved in a central nervous system development and have not been previously identified as candidate genes in neurodevelopmental disorders.
Neurodevelopmental disorders have been ascribed to CNVs of some of the other regions containing GABAA receptor gene clusters. Maternally inherited 15q11-13 duplication is a well described cause of neurodevelopmental phenotypes similar to those in our family, including developmental, speech and motor delays, autism or ASD, seizures, ADD/ADHD, ODD, self-injury and tantrums.4, 9 Only one case of an individual with a neurodevelopmental disorder involving the GABAA cluster on 6q15 (GABRR1 and GABRR) has been reported.10 This 22-year-old female had intellectual disability, ataxia and severe speech deficiency, with an inversion and a deletion described as 46,XX, del(6)(q13q15),inv(6)(p11.2q15 46,XX, del(6)(q13q15),inv(6)(p11.2q15). We are not aware of any previous reports of CNVs among individuals with a neurodevelopmental disorder in the GABAA cluster at 5q34-35.1 (GABRB2, GABRA6, GABRA1, GABRG2 and GABRP).
Several genetic studies have implicated various GABAA receptor subunit genes in neurodevelopmental disorders. Significant findings have been observed for autism and bipolar disorder in candidate gene association studies for GABAA receptor subunit genes on 4p12 and 15q12,11, 12 and for GABRB1, GABRA4, GABRB3, GABRA5 and GABRR3 in the Wellcome Trust Case Control Consortium genome-wide association analysis of bipolar disorder. Mutations in several GABAA genes are known to cause various epilepsies, including GABRG2 in childhood absence epilepsy or generalized epilepsy (with or without febrile seizures)13, 14 and GABRA1 causing juvenile myoclonic epilepsy.15
The Family A reported here has a very complex pattern of mental health problems, including assortative mating for several couples, which is a recognized phenomenon for affective disorders.16 This may limit the ability to definitely confirm the duplication as a susceptibility factor for neurodevelopmental disorders. However, we are able to trace consistently some form of neurodevelopmental phenotype among those with the duplication through three generations. Most likely, there are other factors affecting the final phenotype, a concept that is becoming more evident with a number of publications noting more than one CNV among individuals with neurodevelopmental phenotypes.17
In summary, we report a family with a duplication of 4p13 to 4p12 that appears to segregate with a variable neurodevelopmental phenotype, and an unrelated individual with autism and ADHD with the same duplication. We surmise this is due to duplication of a GABAA receptor subunit gene cluster. This observation provides support to the hypothesis that alterations in the GABAergic system predispose to a variety of neurodevelopmental disorders, including autism, bipolar disorder and seizures.
Note added in proof
After the article went to proof, the motherin Family B consented to testing. FISH probe showed she aslo has hte duplication.
Acknowledgments
‘Some of the data in this manuscript were obtained from the ISCA Consortium database (www.iscaconsortium.org), which generates this information using NCBI's database of genomic structual variation (dbVar, www.ncbi.nlm.nih.gov/dbvar/), study nstd37. Samples and associated phenotype data were provided by ISCA Consortium member laboratories.'
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Mitchell KJ. The genetics of neurodevelopmental disease. Curr Opin Neurobiol. 2011;21:197–203. doi: 10.1016/j.conb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Mefford HC, Batshaw ML, Hoffman EP. Genomics, intellectual disability, and autism. N Engl J Med. 2012;366:733–743. doi: 10.1056/NEJMra1114194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2010;38:181–191. doi: 10.1016/j.nbd.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma H, Ozaki M, Sato H, Takahashi H. Variation in GABA-A subunit gene copy number in an autistic patient with mosaic 4 p duplication (p12p16) Am J Med Genet B Neuropsychiatr Genet. 2008;147B:973–975. doi: 10.1002/ajmg.b.30663. [DOI] [PubMed] [Google Scholar]
- Vincent JB, Horike SI, Choufani S, et al. An inversion inv(4)(p12-p15.3) in autistic siblings implicates the 4p GABA receptor gene cluster. J Med Genet. 2006;43:429–434. doi: 10.1136/jmg.2005.039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaratnam M, Turk J, Vroegop P. Case report: autistic disorder and chromosomal abnormality 46, XX duplication (4) p12-p13. Eur Child Adolesc Psychiatry. 2000;9:307–311. doi: 10.1007/s007870070035. [DOI] [PubMed] [Google Scholar]
- Pizzarelli R, Cherubini E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011;2011:297153. doi: 10.1155/2011/297153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside RD, Pasion R, Mikhail FM, et al. Microdeletion/microduplication of proximal 15q11.2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay. Hum Genet. 2011;130:517–528. doi: 10.1007/s00439-011-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarge E. A distinctive phenotype associated with an interstitial deletion 6q14 contained within a de novo pericentric inversion 6 (p11.2q15) Cytogenet Cell Genet. 2000;91:192–198. doi: 10.1159/000056843. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, et al. Strong genetic evidence for a selective influence of GABAA receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- Cossette P, Liu L, Brisebois K, et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31:184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Reus VI. Assortative mating in the affective disorders: a systematic review and meta-analysis. Compr Psychiatry. 2001;42:257–262. doi: 10.1053/comp.2001.24575. [DOI] [PubMed] [Google Scholar]
- Coe BP, Girirajan S, Eichler EE. The genetic variability and commonality of neurodevelopmental disease. Am J Med Genet C Semin Med Genet. 2012;160C:118–129. doi: 10.1002/ajmg.c.31327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Feuk L, Duggan GE, Khaja R, Scherer SW. Development of bioinformatics resources for display and analysis of copy number and other structural variants in the human genome. Cytogenet Genome Res. 2006;115:205–214. doi: 10.1159/000095916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.