Fig. 1.
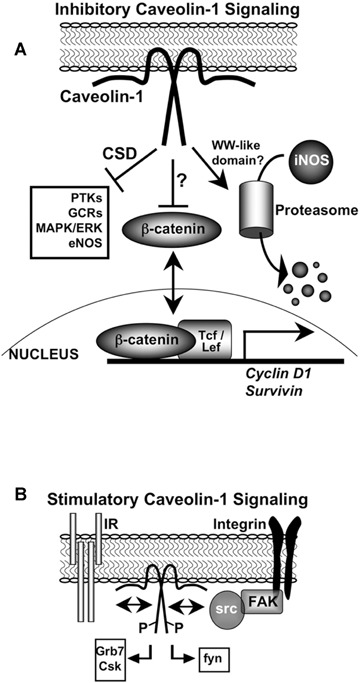
Caveolin-1 is a highly versatile regulator of cell signaling. (A) At the molecular level, Caveolin-1 can modulate the flow of information through different cellular signaling pathways in distinct ways. Caveolin-1 acts as a scaffolding protein by binding to proteins involved in different signal transduction pathways. The caveolin-1 scaffolding domain (CSD) mediates interaction with and inhibition of protein tyrosine kinases (PTKs), G-coupled receptors (GCRs), elements of the MAPK/ERK pathway, members of the protein kinase C (PKC) family and nitric oxide synthases (NOS), in particular the endothelial isoform (eNOS). Additional mechanisms to CSD-mediated inhibition of target proteins that result in loss of target protein activity, include proteasome-mediated degradation as has been described for the inducible isoform of nitric oxide synthase (iNOS). Furthermore, inhibition of β-catenin-Tcf/Lef-dependent transcription of genes such as cyclin D1 and surviving by poorly characterized pathways has been observed. The general consequence of these interactions is that caveolin-1 presence is associated with inhibition of cell proliferation and/or survival. (B) Alternatively, however, caveolin-1 has been implicated as a positive element in insulin receptor (IR) signalling and coupling of the integrin signalling to the MAPK/ERK pathway via focal adhesion kinase (FAK) and src family kinases (SFKs). Such positive signalling downstream of caveolin-1 is frequently associated with phosphorylation on tyrosine 14, whereby downstream positive effectors include Csk, Grb7. Alternatively, however, positive signalling via fyn downstream of integrin receptors is not known to require caveolin-1 phosphorylation. Rather, phosphorylation on tyrosine 14 is implicated as a negative regulator of rac-1 in conjunction with integrin signalling (see text for details).
