Abstract
Rationale
Arteriogenesis is the process of formation of arterial conduits. Its promotion is an attractive therapeutic strategy in occlusive atherosclerotic diseases. Despite the functional and clinical importance of arteriogenesis, the biology of the process is poorly understood. Synectin, a gene previously implicated in the regulation of VEGF signalling, offers a unique opportunity to determine relative contributions of various cell types to arteriogenesis.
Objective
We investigated the cell autonomous effects of a synectin knockout in arterial morphogenesis.
Methods and Results
A “floxed” synectin knock-in mouse line was cross-bred with endothelial (Tie2, Cdh5, Pdg fb) and smooth muscle (Sm-MHC)- specific Cre driver mouse lines to produce cell type specific deletions. Ablation of synectin expression in endothelial but not smooth muscle cells resulted in the presence of developmental arterial morphogenetic defects (smaller size of the arterial tree, reduced number of arterial branches and collaterals) and impaired arteriogenesis in adult mice.
Conclusions
Synectin modulates developmental and adult arteriogenesis in an endothelial cell-autonomous fashion. These findings show for the first time that endothelial cells are central to both developmental and adult arteriogenesis and provide a model for future studies of factors involved in this process.
Keywords: Arteriogenesis, synectin, endothelial cells, VEGF, collateral, VEGF receptor, artery
INTRODUCTION
Arteriogenesis, the process of formation of arterial conduits, is a promising therapeutic approach to the treatment of a number of ischemic vascular diseases. Yet its biology remains poorly understood. New vasculature can form via three distinct processes- vasculogenesis, angiogenesis and arteriogenesis. Vasculogenesis refers to formation of the primitive vascular plexus from vascular progenitor cells during embryonic development. Angiogenesis is the process of new capillary formation that occurs by sprouting or by longitudinal splitting (intussusception) of existing vessels.1, 2 Finally arteriogenesis refers to development of larger vessels such as arterioles and arteries.3 Collateral formation represents a specific case of arteriogenesis. By definition, collateral vessels provide artery-to-artery connections and are thought to play a protective role by providing alternative routes to blood flow.4, 5
While angiogenesis is largely driven by vascular endothelial growth factor (VEGF) production in response to hypoxia6 or inflammation,2 little is known about factors controlling arteriogenesis in general and collateral formation in particular. In adult tissues, arteriogenesis can occur at sites of arterial occlusion where ischemia is not prominent and is thought to be triggered by hemodynamic factors such as changes in shear stress forces sensed by endothelial cells.7, 8 How that happens is the subject of considerable controversy. One possible scenario is that arteriogenesis is the result of remodelling of pre-existing collaterals. Indeed, the existence of small collaterals that expand upon occlusion of the main arterial trunk has been clearly described, both in animals9 and in humans10, and the ability to restore compromised tissue perfusion has been linked to the extent of pre-existing collateral circulation.11
Alternatively, adult arteriogenesis has been described as a de novo process that occurs by the expansion and arterialization of the capillary bed.12–14 In this scenario, the ability of EC to proliferate and to secrete growth factors, such as PDGF, are crucial for the new vascular network development and subsequent arterialization via recruitment of mural cells. One piece of evidence strongly in favour of this hypothesis is the extent of new arterial growth observed by micro-CT angiography following a large artery occlusion.15 This is highly unlikely to arise solely from pre-existing collaterals.
Studies over last decade have identified a number of growth factors, including PDGF, FGF and VEGF16–20, cytokines such as MCP121, 22, peptides such as NPY23 and master regulators such as HIF-1α24, 25, HIF-2α26 and PR3927, 28 that can promote arteriogenesis. Of these, VEGF-A appears to play the central role. In particular, experimental studies have demonstrated that collateral growth is prevented by anti-VEGF-A neutralizing antibodies5, VEGF receptor inhibitors29, and soluble VEGFR traps30, while genetic approaches further demonstrated the requirement for VEGF-A expression for collateral development in healthy tissue.17 Disruption of VEGF signalling due to impairment of VEGFR2 trafficking has also been shown to result in decreased arteriogenesis.31–33
Although the role of VEGF-A in arteriogenesis is well established, its cellular site of action remains uncertain. In this study we set out to address the cellular underpinnings of VEGF-dependent arteriogenesis. To this end, we have taken advantage of the recent identification by our laboratory of an arterial morphogenetic defect associated with a global deletion of synectin.31, 32 Synectin is a widely expressed single PDZ domain protein that interacts with a variety of plasma membrane and cytoplasmic molecules31, 34, 35 to control intracellular signalling. Homozygous disruption of synectin in mice or a knockdown of its expression in zebrafish result in a selective reduction of arterial morphogenesis including decreased branching and reduced size and diameter of the arterial vasculature.31 Synectin-null endothelial cells have reduced responsiveness to VEGF stimulation31, 32 and decreased activation of ERK, while their responses to other growth factors such as FGF and IGF are normal.32 In addition, synectin null mice display downregulation of PDGF expression in the endothelium, likely accounting for the loss of vascular smooth muscle cells coverage of smaller blood vessels observed in these settings.36 This gene, therefore, offers a unique ability to help determine relative contributions of various cell types to arteriogenesis.
To study that problem, we generated a mouse line with a floxed synectin gene knocked-in into the synectin (gipc1) locus and cross-bred it with endothelial (Tie2-, Cdh5- and Pdgfb-) and smooth muscle (Sm-MHC-) Cre mouse lines to produce endothelial (EC) and smooth muscle cell (SMC)- specific deletions, respectively. We find that ablation of synectin expression in EC but not SMC mimics the arterial phenotype of global synectin null mutants31 thereby implicating endothelium as the key cell type regulating arteriogenesis.
METHODS
Full methods are described in the online data supplement.
Mice
Mice homozygous for a conditional allele of synectin (gipc1) were generated by flanking exon 2 with loxP sites. Exon 2 of the synectin (gipc1) was deleted by crossing the gipc1flox/flox mice with either the Sm-MHC-Cre37, Tie2-Cre38, Cdh5-CreERT239 or Pdgfb-Cre/ERT240 mice. All animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Yale University.
Primary smooth muscle and endothelial cell isolation and culture
Primary SMCs were isolated from dorsal aorta, as previously described41 with minor modifications. Primary ECs were isolated from the heart and lung of adult mice using a previously described protocol.31
Hindlimb ischemia model
This was done as previously described by our lab.28, 31 Laser-Doppler flow- imaging was carried out using a Moor Infrared Laser Doppler Imager (LDI; Moor Instruments Ltd) at 36.5°C– 37.5°C under isofluorane anesthesia.
Micro-CT angiography
Microcomputed tomography (mCT) of the cardiac, renal and hindlimb vasculature was done by injecting 0.7ml bismuth contrast solution in the descending aorta and the vasculature was imaged and quantified as described previously.28, 31, 42
Spino-trapezius assay
Spinotrapezius muscles (both left and right) from euthanized mice, were stripped of fascia and dissected as described previously.14, 43 Quantification of vessel diameters and collateral arcades was done using ImageJ software.44
Retina analysis
The eyes were harvested at postnatal day 5 (P5) and 17 (P17) and processed as described in Supplementary Data.
In vivo Matrigel assay
The Matrigel was pre-mixed with heparin (5U) with or without VEGF-A165 (100ng/ml) and injected subcutaneously. 7 days later the plugs were recovered from the sacrificed mice, embedded in OCT, cryosectioned in 10 µm sections and stained with anti-CD31 antibody.
Wound healing assay
Wound healing assays were done by creating in the skin of the back of 12–14 week-old male mice 6mm punch wounds. The healing process was analyzed over time.45
RESULTS
Generation of gipc1 floxed mice and smooth muscle and endothelial knockouts
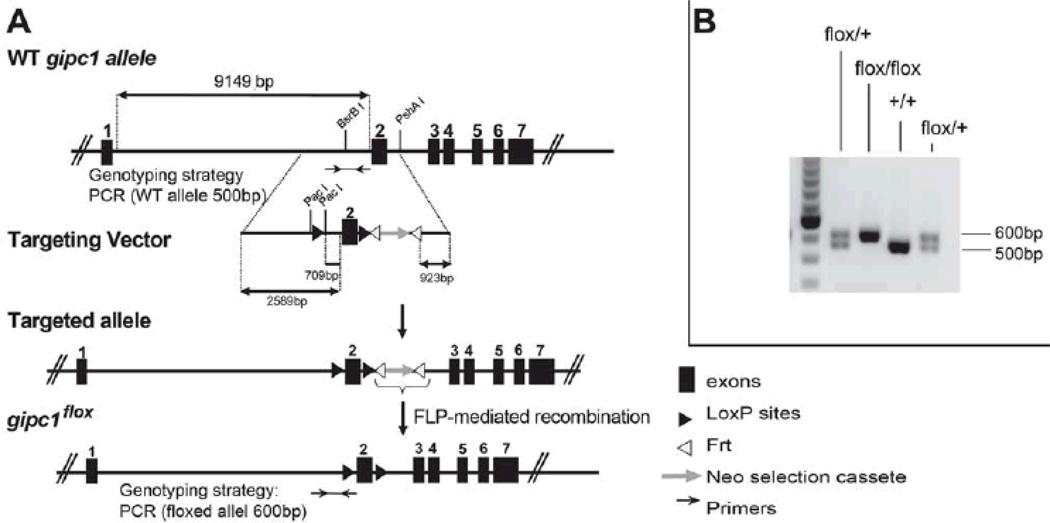
To evaluate the specific contributions of EC and SMC to arteriogenesis, we generated mice carrying a floxed synectin allele inserted into the endogenous gipc1 gene locus. The targeting strategy (Fig. 1A) resulted in the creation of a gipc1 allele with loxP sites flanking a neomycin resistance cassette and exon 2 of the gipc1 gene. The neomycin resistance gene was subsequently removed using flp- mediated recombination. Screening of the progeny confirmed the presence of the floxed allele (Fig. 1B).
Figure 1. Generation of a floxed allele of the mouse Synectin gene.
(A) The targeting vector contains: a loxP site (black triangle) into BsrB I restriction site upstream of exon 2, exon 2 cDNA ,a fragment with Neo selection cassette (grey arrow) and a loxP site into the PshAI site downstream of exon 2. The Frt-flanked Neo-cassette was removed by Flp-mediated, site-specific recombination (Frt recombination elements are white triangles). (B) Genomic mouse DNA was genotyped with the oligonucleotides indicated in A (black arrows).
In order to ablate synectin expression in SMCs, gipc1fl/fl mice were crossed with Sm-MHC-Cre strain and the progeny was bred to produce mice homozygous for the loss of smooth muscle synectin expression (SynSMKO). Genotyping of litters containing SynSMKO mice (Sm-MHC-Cre;SynFF) revealed the presence of all genotypes with the expected Mendelian frequency.
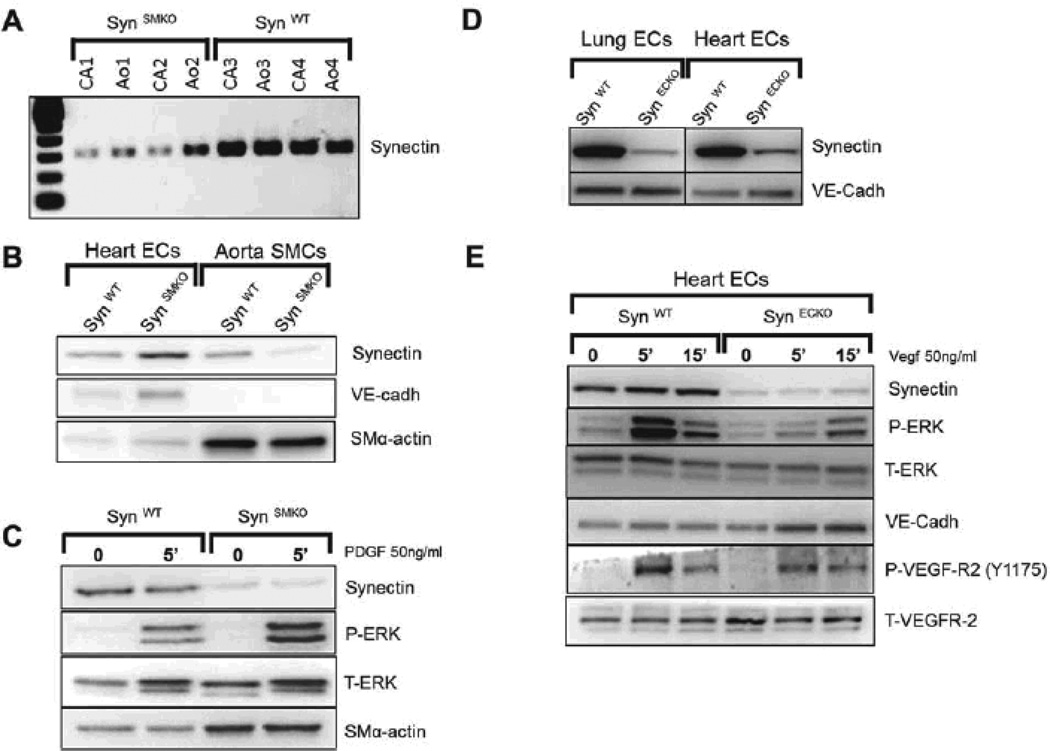
Carotid arteries and aorta from SynSMKO mice had markedly lower mRNA levels of synectin than littermate controls (Fig. 2A). Western blot analysis of synectin expression in primary SMC and EC, isolated from these mice confirmed nearly complete loss of synectin expression in SMC but not EC (Fig. 2B). Stimulation of SynSMKO SMC with PDGF-BB, one of the key growth factors regulating SMC migration and proliferation, revealed no differences in ERK activation as compared with control SMC, demonstrating that synectin expression in smooth muscle is not required for a normal response to PDGF-BB stimulation (Fig. 2C). These observations are in line with what has been previously shown in SMC from global synectin null mice.36
Figure 2. Validation of SynSMKO and SynECKO mouse lines.
(A–C) In vivo ablation of synectin expression in smooth muscle cells (SynSMKO). (A) Decreased synectin RNA levels in carotid (CA) and aorta (Ao) from SynSMKO mice compared with control littermates. (B) Western blot of total cell lysates of aortic smooth muscle (SMC) and heart endothelial cells (EC) from SynSMKO mice. Note a reduction in synectin protein levels in SMC but not in EC. (C) ERK activation: Western blot analysis of total cell lysate of aortic SMC. Confluent, serum starved cells were stimulated for 5 min with 50ng/ml PDGF-BB. Phosphorylation of ERK (P-ERK) in response to PDGF-BB is not altered in synectin null smooth muscle cells (SynSMKO) relative to wild type smooth muscle cells (SynWT). T-ERK: total ERK. SM-α-actin: SMC α-actin. (D, E) In vivo ablation of synectin expression in endothelial cells (SynECKO).(D) Decreased synectin protein levels in endothelial cells isolated from SynECKO mice compared with SynWT mice. (E) Western blotting of total cell lysates isolated from heart endothelial cells (EC). Confluent, serum starved EC were stimulated for the times indicated with 50 ng/ml VEGF-A165. Note reduced phosphorylation of ERK (P-ERK) and in VEGFR2 Y1175 site in synectin null endothelial cells (SynECKO).
In order to ablate synectin in EC, homozygous floxed synectin mice were crossed with the Tie2-Cre mouse line and the progeny was bred for endothelial-specific knockout of synectin (SynECKO). Western blot analysis of primary heart and lung EC isolated from these confirmed a marked reduction in synectin expression in these cells (Fig. 2D). Previous studies from our laboratory have demonstrated reduced responsiveness to VEGF stimulation of synectin null arterial EC isolated from synectin global knockout mice.31, 32 To confirm that EC-selective disruption of synectin resulted in the same phenotype at the cellular level, we examined VEGF signaling in ECs isolated from SynECKO mice. Analysis of VEGF receptor 2 (VEGFR2) activation following stimulation with VEGF-A demonstrates an expected reduction in Y1175 site phosphorylation and a decrease in ERK1/2 activation (Fig. 2E).
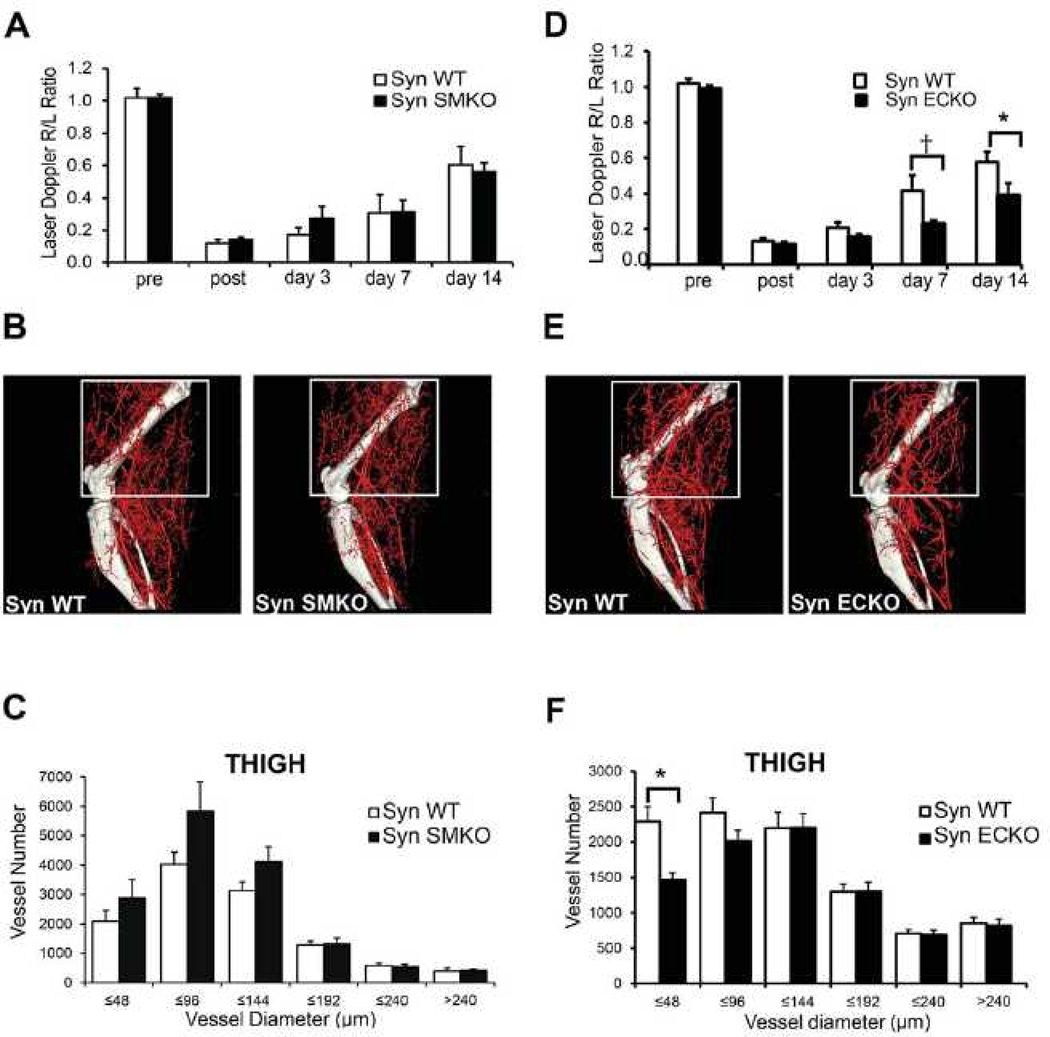
Impaired arterial development in SynECKO but not in SynSMKO mice
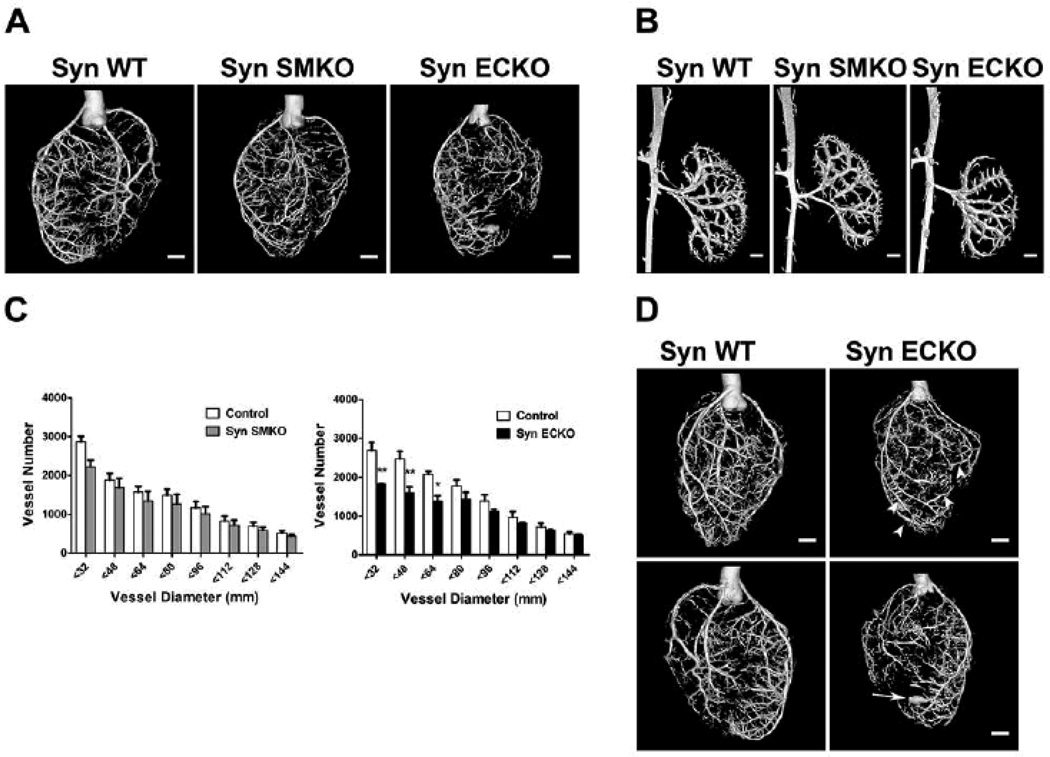
In order to assess the impact of EC and SMC synectin deletion in the arterial development, we performed micro-computed (mCT) angiography of the heart (Fig. 3A) and renal vasculature (Fig. 3B) of SynSMKO, SynECKO and littermate control mice as described previously.28, 31 Arterial trees in both the heart and kidneys of SynECKO, but not SynSMKO mice, exhibited less branching, had fewer artery-to-artery connections (collateral arteries) and appeared smaller in diameter. Quantitative analysis of coronary circulation showed a significant decrease in the number of smaller arteries (≤64µm in diameter) in SynECKO mice. SynSMKO mice showed a trend towards reduced number of smaller arteries (≤32µm in diameter) that was not statistically significant (Fig. 3C). This reduction in the number of smaller caliber arteries in SynECKO mice is very similar to that observed in the global synectin knockout.31 To our surprise we found aneurysmal dilations of distal coronary arteries (Fig. 3D, arrowheads) and even frank tubular aneurysms in SynECKO mice (arrow) that were not previously seen in global synectin null mice.
Figure 3. Developmental arteriogenesis defects are present in SynECKO but not in SynSMKO mice.
Representative reconstructed mCT images of whole hearts (A) and kidneys (B) arterial vasculature (16µm resolution; n=4) from age- and gender-matched SynWT, SynSMKO and SynECKO mice. Note marked reduction in branching in SynECKO mice compared with SynSMKO or SynWT control mice. (C) Quantitative analysis of mCT images of whole hearts (grey bars, SynSMKO and black bars SynECKO). Note a marked decrease in total number of <64µm diameter arterial vessels in SynECKO mice relative to control littermates.): F = 3.962, p = 0.003; post-hoc Tukey's HSD test: * p<0.01; ** p<0.001. (D) Reconstructed mCT images of the heart vasculature from SynECKO mice. Note the presence of aneurism-like structures (arrow) and increased diameters in the distal part of some arteries compared with their proximal partly (arrowheads). Scale bar 1mm.
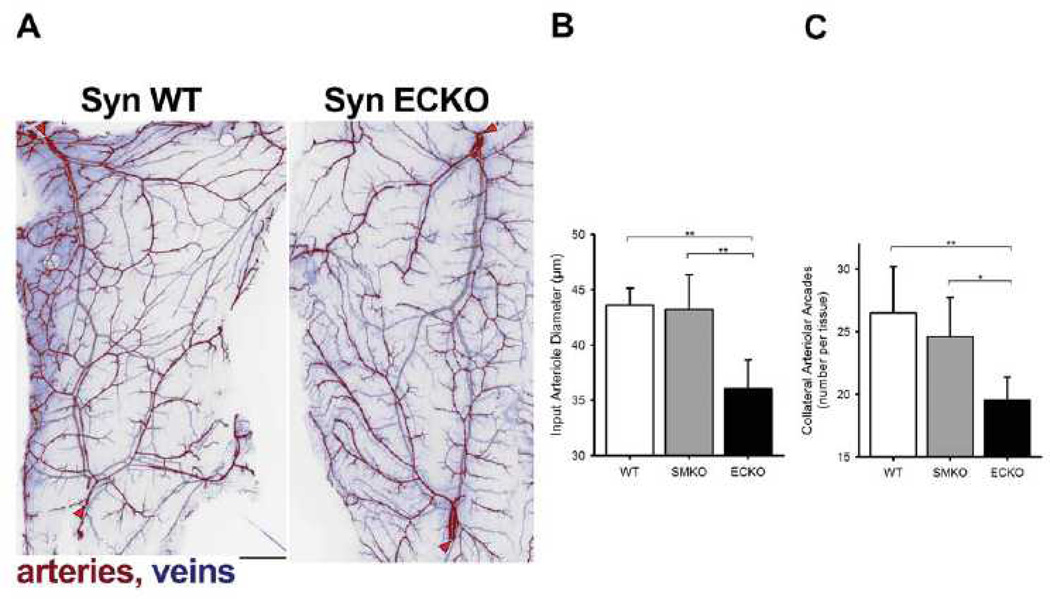
To gain a better understanding of arterial microcirculation changes associated with an endothelial-specific synectin knockout, we examined vascular networks in the spino-trapezius muscle.14 Because of its thinness (~150 µm), the entire vascular network of the muscle can be imaged in whole-mount at micron-level resolution (Fig. 4A). The muscle is fed by multiple arterioles, notably lateral and caudal feed vessels (red arrowheads, Fig. 4A) which are connected by an arteriolar arcade network providing multiple collateral pathways for blood flow.14, 46 In wild type mice, the collateral networks have a ramified arcade structure, with multiple arteriole linkage while in SynECKO mice the network has a dendritic structure with smaller arteries and fewer arteriolar-level connections (Fig. 4A). No abnormalities were observed in SynSMKO mice (images not shown). This finding was confirmed by quantitative analysis of the spino-trapezius circulation in wild type, SynECKO and SynSMKO mice (Fig. 4B, C). The input arterioles are significantly narrower in the SynECKO but not SynSMKO mice compared to littermate controls (Fig. 4B), thus confirming spino-trapezius angiography and coronary mCT observations. In addition, the number of arteriolar arcades (collateral arteries) is significantly lower in SynECKO as compared with SynSMKO or littermate control mice (Fig. 4C).
Figure 4. Quantification of arterial networks in mouse skeletal muscle.
(A) Fluorescent image of the caudal half of the mouse spinotrapezius. Pseudocoloring is used to indicate arteries (red) and veins (blue). Scale bar, 500 µm. Input arterioles entering the muscle are denoted by red arrowheads. Note extensive arcading arterial networks providing collateral pathways between the lateral (top left) and caudal (bottom left) input arterioles. The networks of spinotrapezius muscle vasculature in controls (SynWT) have a ramified arcade structure, with multiple arteriole arteriole linkage. Note that networks in SynECKO mice have a predominatly denditric structure with few arteriolar-level connections. (B) Diameter of the input arterioles as they enter the spinotrapezius. (C) Number of arcades in the caudal collateral spinotrapezius arterial network. Wild type mice, n=10; SynSMKO and ECKO mice, n=6. ANOVA with Tukey’s HSD post-hoc test analysis: *, p<0.05; **, p<0.01.
Postnatal arteriogenesis defects in synectin endothelial knockout mice
To investigate the effect of SMC- and EC-specific synectin knockout on arterial morphogenesis in adult tissues, we next analysed arteriogenesis in SynSMKO and SynECKO mice using a hindlimb ischemia model. In this model, arteriogenesis is induced by ligation and excision of a segment of the common femoral artery of 10–12 week old mice.28 Following artery ligation, blood flow in the distal limb is assessed by a deep penetrating Laser Doppler and expressed as a ratio of flow in the foot of operated to non-manipulated limb. Immediately after surgery, there was a dramatic but equal reduction in perfusion in all three groups (Fig. 5A, D). Blood flow in the distal limb of control and SynSMKO mice recovered to the same extent over the next 2 weeks (Fig. 5A). As growth of new arterial vasculature is the principal event responsible for blood flow restoration in this model, we next performed mCT analysis to visualize and quantify the extent of hindlimb vasculature, 14 days after surgery. In agreement with the finding of unimpaired distal blood flow recovery, mCT of the arterial vasculature confirmed comparable arteriogenesis in SynSMKO and wild type mice (Fig. 5B, C). Interestingly, there was a non-significant trend towards larger artery sizes in SynSMKO than control mice (Fig. 5C). On the other hand, blood flow recovery was significantly impaired in SynECKO mice, which was apparent one week after surgery (Fig. 5D) and was similar to that previously observed in synectin global knockout mice. As expected, mCT analysis of the arterial circulation demonstrated a significant reduction in the number of smaller arterial vessels in the tight muscle (Fig. 5E, F).
Figure 5. Impaired blood flow recovery in SynECKO mice following ligation of femoral artery.
(A, D) Laser Doppler analysis of blood flow perfusion: SynSMKO (A) and SynECKO (D) mice were subjected to common femoral artery ligation. The graph shows blood flow in the ischemic foot (right) expressed as a ratio to flow in the normal foot (left) (R/L) at various time points after femoral artery ligation. Note the absence of flow recovery in SynECKO mice at 7 and 14 days. Mean±SD, *p<0.05, †p=0.07, n=10 per group. (B, E) Representative mCT images of reconstructed limb arterial vasculature from SynSMKO (B) and SynECKO (E) compared with control littermates 14 days after surgery (C, F) Quantitative mCT analysis in SynSMKO (C) and SynECKO (F) compared with control littermates. Note a significant decrease in the amount of smaller size arteries in SynECKO mice compared with control group (C) *p<0.05, n=5 mice/group.
Inducible ablation of synectin expression in endothelial cells
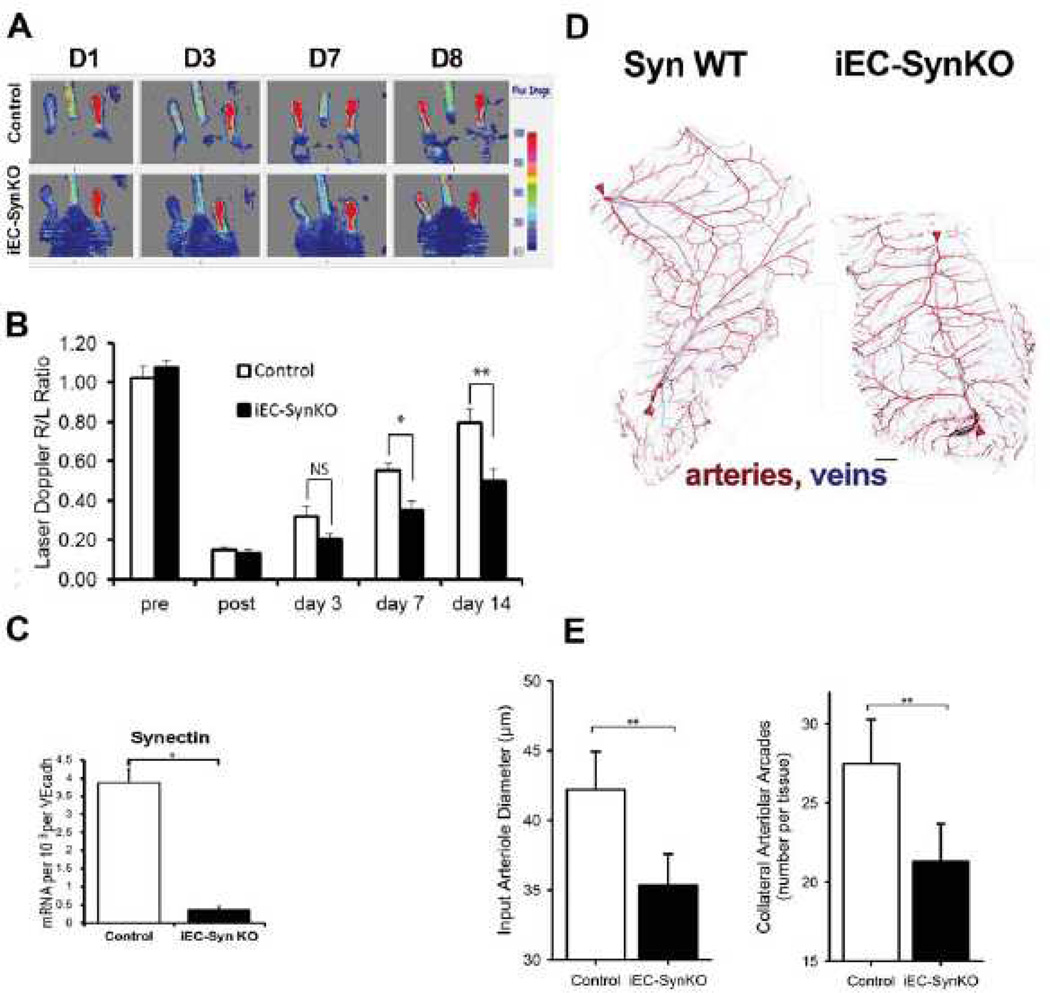
Since the Tie2 promoter has been shown to activate Cre expression not only in endothelial lineage but also in hematopoietic lineages during development,47 this may result in deletion of synectin expression in monocytes and monocyte-derived macrophages. Indeed, these cells play an important role in postnatal arteriogenesis, particularly, in the hind limb ischemia model.48, 49 To exclude the possibility that decreased synectin expression in cells other than the endothelium was responsible for the impaired arteriogenesis following common femoral artery ligation in adult mice, we employed Cdh5-CreERT2 transgenic mouse line.39 Unlike Tie2, Cdh5 expression is much more restricted to the endothelium and very little, if any, expression occurs in cells of hematopoietic lineages postnatally.
Analysis of the arteriogenesis in the hindlimb ischemia model was done in 10 weeks gender matched iEC-SynKO mice and controls (n=6/group). Similar to SynECKO (Tie2-Cre; SynFF), iEC-SynKO (Cdh5-CreERT2;SynFF) mice demonstrated a significant decline in blood flow recovery following the common femoral artery ligation (Fig. 6A, B). Quantitative PCR analysis of synectin mRNA expression in lung EC isolated from iEC-SynKO mice (n=3) showed markedly decreased expression compared with controls (Fig. 6C).
Figure 6. Arteriogenesis defects in iEC-SynKO mice.
(A, B) Laser Doppler analysis of blood flow perfusion in iEC-SynKO (Cdh5-CreERT2;SynFF) and control mice subjected to common femoral artery ligation. (B) Laser-Doppler analysis of blood flow recovery after surgery expressed as a ratio of flow in ischemic (R) to normal (L) foot (R/L ratio) at various time points. Note a significant decrease in flow recovery in iEC-SynKO mice at 7 and 14 days compared to controls. Mean±SD, (*p≤ 0.05, **p≤ 0.01, n=6 per group). (C) Decreased synectin RNA levels in lung EC from iEC-SynKO mice compared with control. (D) Image of the caudal half of the mouse spinotrapezius. Staining for smooth muscle α-actin has been pseudo-colored to indicate arteries (red) and veins (blue). Scale bar, 500 µm. Input arterioles entering the muscle are denoted by red arrowheads. (E) Quantitative analysis of the number of collateral arcades in the spino-trapezius muscle and diameter of the input arterioles as they enter the muscle in iEC-SynKO and WT mice (n=3 each); p≤0.05, two-tailed t-test.
Analysis of the spino-trapezius vascular networks in iEC-SynKO mice again demonstrated a significant reduction in the number of collateral arcade structures compared with control littermates (Fig 6D, E). In addition, diameters of the input arterioles were significantly less in iEC-SynKO mice compared with control littermates (Fig. 6E), similarly to what was observed in SynECKO mice.
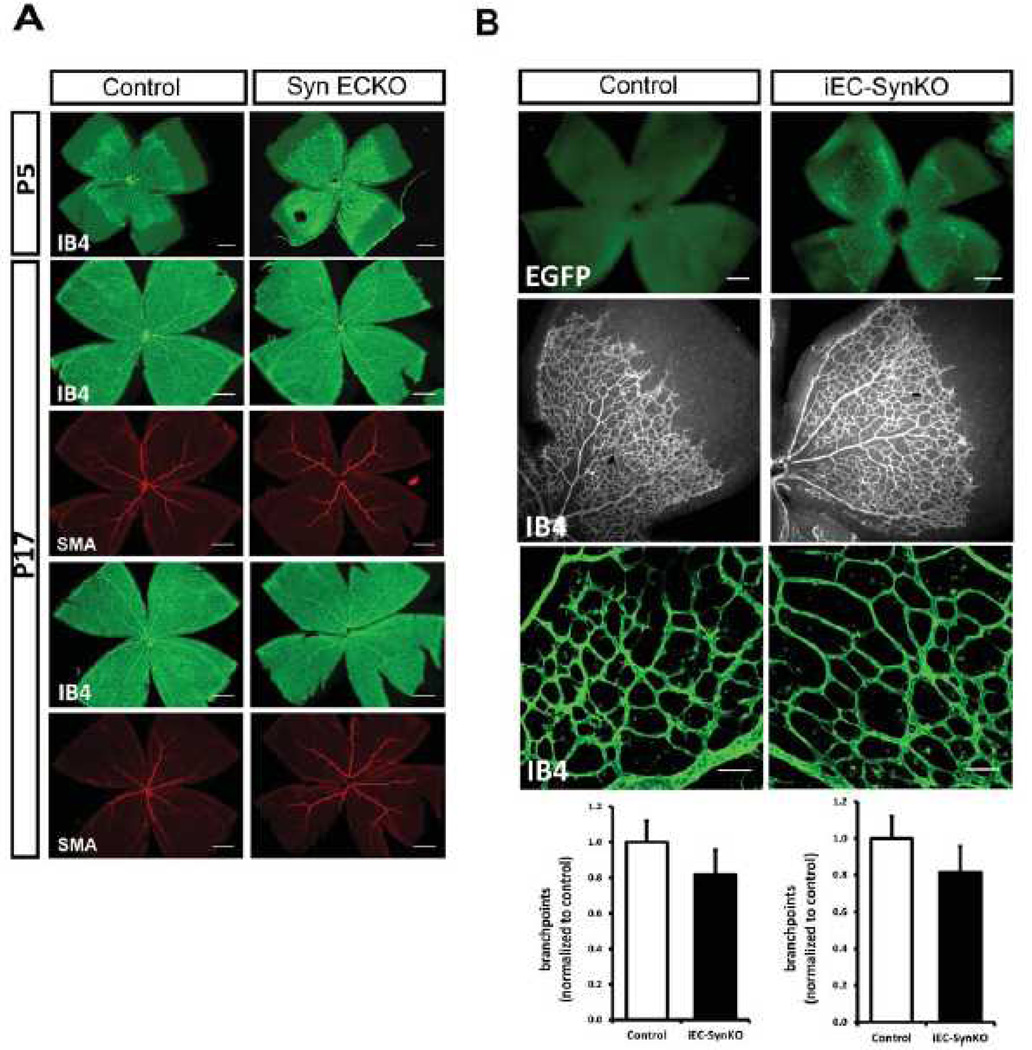
To assess the impact of reduced endothelial synectin expression in angiogenesis, we analysed P5 and P17 retinas from Tie2-Cre;SynFF (SynECKO) mice (Fig 7A) and P5 retinas from Pdgfb-iCre/ERT2;SynFF (iEC-ECKO) mice (Fig 7B) induced on the first postnatal day. Retinal angiogenesis, included the extent of vascular coverage and the number of vascular branchpoints was the same in both synectin mutant lines and in their littermate controls.
Figure 7. Retinal angiogenesis is not affected in SynECKO and iEC-SynKO mouse lines.
(A) Whole mounts of P5 and P17 retinas from SynECKO mice labelled with isolectin B4 (IB4) and stained with smooth muscle α-actin (SMA) antibody. Representative images for P5 (top) and P17 (two, bottom) are shown. There were no visual differences in vessel area and SMC coverage between SynECKO mutants and controls. Scale bar 0.5mm. n=4 mice/group. (B) Quantitative analysis of P5 whole mount retinas from iEC-SynKO mice generated with Pdgfb-iCre. Expression of EGFP in mutant pups demonstrates the efficiency of Cre induction after tamoxifen administration (top, scale bar 200 µm). Retinal vasculature and quantitative analysis of vascular area and vessel branchpoints are shown (bottom, scale bar 50 µm, N=3 mice/group.
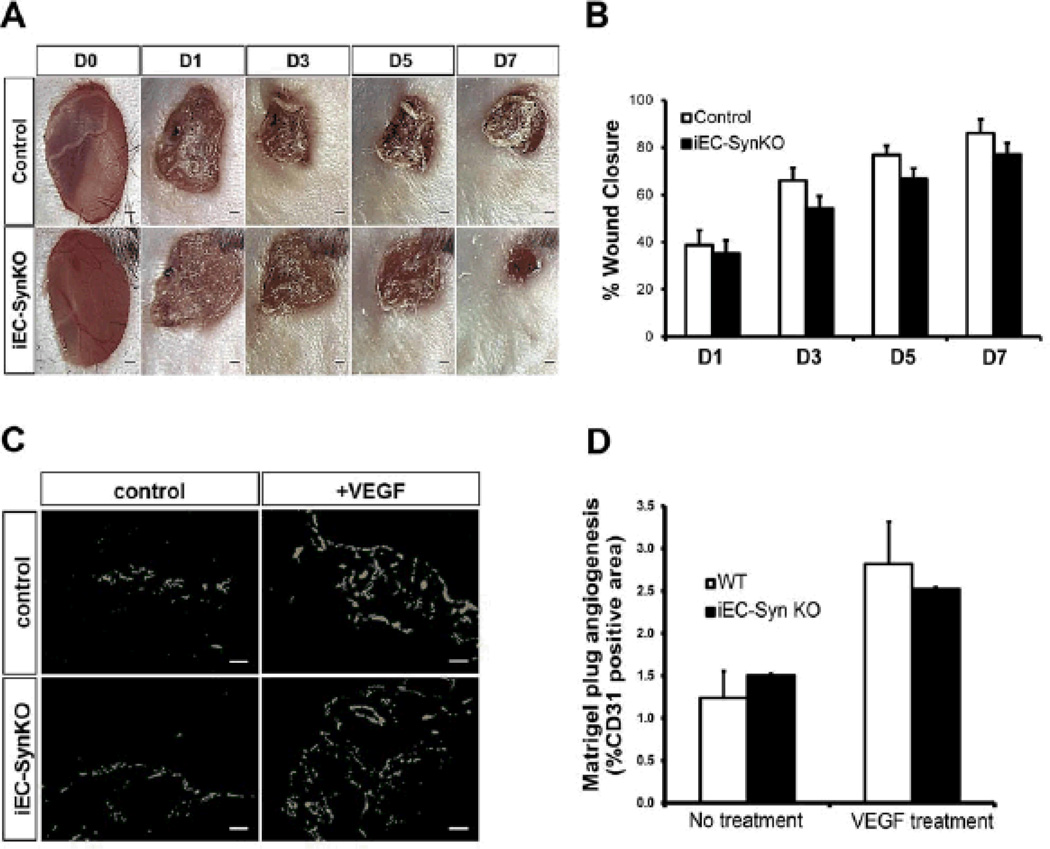
To address the effect of synectin ablation on adult angiogenesis, we first utilized a puncture wound model in Cdh5Cre-ERT2; SynFF mice (iEC-ECKO). The rate of wound healing in this model reflects the underlying angiogenesis and angiogenic defects impair the rate of wound closure.45 Visual inspection and quantitative analysis demonstrated no difference in wound closure rate between synectin deletants and littermate controls (Fig 7A, B). Finally, iEC-SynKO mice were utilized for an in vivo Matrigel assay. Analysis of Matrigel plugs impregnated with VEGF extracted from control and iEC-SynKO mice demonstrated no differences (Fig 7C, D).
DISCUSSION
The results of this study demonstrate that synectin modulates developmental and adult arteriogenesis in an endothelial cell-autonomous fashion. In addition, we demonstrate that endothelial synectin is not required for adult angiogenesis. Specifically, synectin knockout in EC results in a number of developmental and adult arterial morphogenetic defects including smaller size of the arterial tree, reduced numbers of arterial branches and collaterals, and impaired blood flow recovery after common femoral artery ligation. At the same time, SMC-specific deletion of synectin does not affect the size of the arterial tree, the number of collaterals, or blood flow recovery in adult animals.
We focused on cell-type-specific synectin knockout because of its critical role in the regulation of VEGF signalling, which is central to the process of arteriogenesis.50 Synectin accomplishes this by facilitating, in complex with myosin-VI, trafficking of newly endocytosed VEGFR2-containing vesicles away from the plasma membrane to EEA1+ endosomes. This allows prolonged phosphorylation of the VEGFR2 Y1175 site involved in activation of PLCγ/ERK signalling which is essential for normal arteriogenesis.51 While this mechanism has been shown to operate in EC, given the ubiquitous nature of synectin expression and the central role of synectin/myosin-VI complex in trafficking intracellular vesicles formed during clathrin-dependent endocytosis52, it is possible that the same holds true in all cells expressing VEGF receptors.
To address, therefore, the cell autonomous effects of synectin, we used SMC- and EC-specific Cre driver lines crossed with floxed synectin knock-in mice to generate synectin deletions in these cell types. Analysis of arterial circulation in hearts, kidneys and skeletal muscles of endothelial cell-specific synectin mice demonstrated reduced numbers of arteries, reduced branching and decreased arterial diameter, while no significant differences in these parameters were seen in smooth muscle-specific synectin knockout mice. These results were confirmed by analysis of arteriolar networks in the spino-trapezius muscle where we also observed reduced arteriolar size and fewer artery-to-artery connections in EC-but not in SMC-specific synectin knockouts. Adult arteriogenesis, assessed as the ability to form new arteries in response to common femoral artery ligation, was also abnormal in SynECKO but not SynSMKO mice. This was demonstrated both by the impaired blood flow recovery to the distal limb in SynECKO mice and by the reduced numbers of small arteries forming above the knee.
All of these studies were done using Tie2-Cre driver line which, in addition to EC, is also expressed in a small number of hematopoietic precursor cells which differentiate into a number of cell types, including monocytes/macrophages that play an important role in arterial morphogenesis. To exclude the possibility that Tie2 expression in cells other than EC was responsible for the observed arteriogenesis defects, floxed synectin mice were also crossed with Cdh5-CreERT2 transgenic mouse line that has a much more endothelium-restricted expression. Activation of Cdh5-driven Cre (iEC-SynKO) either during development or postnatally reproduced the arterial morphogenesis defects observed in global synectin null and in SynECKO mice while angiogenesis (developmental and adult) was not affected.
As already alluded to, synectin regulates VEGFR2 signaling and, specifically, activation of ERK, by ensuring, in partnership with neuropilin-1 and myosin-VI, a rapid transit of VEGFR2 through the “phosphatase zone” thus preventing its deactivation by PTP1b.33, 53 Any reduction in endothelial ERK activation, as seen in synectin or myosin-VI knockouts31, 32 or in Nrp1cyto knock-in mice,33 results in arterial morphogenic defects. Interestingly, in all of these cases, as well as in the present study of endothelial-specific synectin deletion, angiogenesis remains normal.
The availability of synectin floxed mice has allowed us to address another critical point in arteriogenesis biology- namely the identification of the cell type critical to this process. The fact that endothelial disruption of synectin expression impairs all forms of arterial morphogenesis- developmental and adult as well as collateral development, clearly states that endothelial activation of ERK signaling, a process that synectin regulates, is central to arteriogenesis.
The nature of arteriogenic process, especially in adult tissues, has longed remained poorly understood. The two most frequently advanced hypotheses are the remodelling of pre-existing arterial vasculature into larger size arteries and de novo growth of new arteries. The remodelling of pre-existing arteries (usually invoked in terms of collateral development, a special case of arteriogenesis) implies the expansion of the lumen and increased size of the vessel wall, a process that requires SMC proliferation.19, 54 In contrast, the de novo growth of new arteries suggests formation of new arterial conduits via arterialization of the capillary network. Such a process would be expected to be heavily dependent on EC proliferation since it requires capillary bed expansion.12–14
Defective arteriogenesis in EC-specific synectin mutants strongly argues for the second hypothesis. The decreased VEGF responsiveness of synectin-deficient endothelium likely affects arteriogenesis by affecting two critical process- formation and expansion of vascular lumen and recruitment of mural cells. With regard to the former it is interesting to note that all cases of reduced endothelial ERK activation31–33, including the present study of EC-specific synectin deletion, arterial diameter for comparable arteries (e.g. proximal coronary vs. proximal coronary) is smaller than in control mice. Furthermore, in vitro assay demonstrate that reduced ERK activation leads to reduced lumen formation33. At the same time, excessive endothelial activation of ERK signaling leads to formation of larger than normal diameter arteries.55
With regard to the latter, decreased endothelial synectin expression leads to reduced secretion of PDGF-BB36, growth factor most associated with mural cell recruitment.56, 57 Combined, these two defects would lead to decreased capillary bed arterialization, a process that requires an increase in capillary lumen diameter and mural cell recruitment.
The role of synectin and endothelial VEGF resistance is supported by two other studies: 1) rescue of synectin null arteriogenic defect by treatment with PTP1b inhibitor; which abolishes the delayed VEGFR2 trafficking thereby activating VEGR2/ERK signaling31, 32, and 2) rescue of synectin null and hypercholesterolemic mice (which are VEGF resistant) by treatment with a PI3K inhibitor, which activates ERK signaling independently of VEGFR2.58 While these studies were done with global knockouts or globally impaired mice (hypercholesterolemia), the present study, with a selective endothelial knockout, closes the loop. Impaired VEGF signalling only in endothelial cells is enough to affect arteriogenesis. This supports the de novo arteriogenesis rather than remodelling of pre-existing collaterals, as the dominant process.
In addition, we employed a severe model of hindlimb ischemia that effectively eliminates any protective role of pre-existing collaterals. In fact, measurements of blood flow immediately after surgery demonstrate no difference between WT and synectin EC-null mice (iEC-SynKO, SynECKO), supporting this argument. Also, the difference in blood flow recovery does not appear until day 7, a time course that is consistent with capillary bed arterialization which is largely endothelium-driven, while pre-existing collateral growth typically peaks by day 3.28, 59 Therefore, all subsequent differences are largely due to de novo arteriogenesis, not to pre-existing collateral remodelling.
More broadly, endothelial resistance to VEGF signalling, whether due to defective VEGFR2 trafficking or to other mechanism(s), would be expected to result in arteriogenic defects. This concept is supported by a number of previous studies that addressed the role of VEGF signalling in the process of collateral formation.5, 17, 29, 30
In conclusion, understanding how new arteries form in response to ischemia is crucial to developing effective treatments in ischemic vascular diseases. This study identifies endothelial cells and endothelial VEGF responsiveness as key elements in growth and development of new arteries. We suggest that the focus of future therapeutics efforts should be on overcoming endothelial VEGF resistance frequently observed in patients with underlying pathologies such as atherosclerosis28 and diabetes60, 61 and that simply providing additional VEGF either directly or via stimulation of mononuclear cell recruitment, is unlikely to result in a therapeutic benefit.
Supplementary Material
Figure 8. Adult angiogenesis is not affected in iEC-SynKO mouse.
(A,B) Wounds were introduced in iEC-SynKO (Cdh5-Cre-ERT2) and littermates controls and imaged at the indicated times. Representative images of healing wounds are shown in (A) and quantification of the area of wound closure (mean ±SEM) are shown in (B). n=4 mice/group. (C,D) Matrigel implants from iEC-SynKO (Cdh5-Cre-ERT2) and littermates controls. Representative images (C) and quantification (D) from CD31 imunolabelled Matrigel plug sections, with and without VEGF-A165 treatment. Scale bar 100um. n=3 mice/group
NOVELTY AND SIGNIFICANCE.
What Is Known?
Arterial occlusion results in the failure of blood delivery, leading to tissue ischemia.
In some circumstances blood flow can be re-established via the development of collateral vessels.
Formation and growth of new arteries and collateral vessels are poorly understood processes.
What New Information Does This Article Contribute?
Partial disruption of vascular endothelial growth factor receptor 2 (VEGFR2) signaling in endothelial cells impairs arteriogenesis, indicating that endothelial cells play a central role in arteriogenesis.
These findings suggest a model of the de novo arteriogenesis, in which arteries are formed by arterialization of the capillary bed, expansion of the vascular lumen, and recruitment of mural cells to form new arterial structures. This process is driven by endothelial cell responses to VEGF.
Adult arteriogenesis is a physiologically important but poorly understood process. We examined the role of the endothelium in regulating arteriogenesis by specifically deleting synectin (gipc1), a protein involved in the regulation of VEGFR2 trafficking in endothelial and smooth muscle cells. We found that endotheliumspecific deletion of synectin decreased arterial formation during embryonic development and reduced collateral formation in adult tissues. Together with prior studies that have demonstrated synectin’s involvement in VEGFR2 trafficking and activation of ERK signalling, our observations point to the key role of endothelium and, specifically, endothelial ERK signalling, in regulating arteriogenesis. These results suggest that impaired endothelial responsiveness to VEGF, during conditions such as diabetes or atherosclerosis, would attenuate arteriogenesis.
ACKNOWLEDGMENTS
We thank Steve N. Fiering (Dartmouth knockout core lab) for his help with generation of gipc1flox/flox mice, Rita Webber, Nicole Copeland and Wayne D. Evangelisti (YCVRC) for mouse colony management, Jiang Yifeng for mCT angiography, John Bent for help with image editing and Roa Harb for assistance with statistical analysis.
SOURCES OF FUNDING
Supported, in part, by Brown-Coxe Foundation postdoctoral fellowship (FM), NIH grant HL62289 (MS), Leducq ARTEMIS Trans-Atlantic network grant (FM, MS) and American Heart Association Beginning Grant-in-Aid 12BGIA12060154 (FMG).
Nonstandard abbreviations and Acronyms
- EAA1
Early Endosome Antigen1
- EC
Endothelial cell
- ECKO
Endothelial cell conditional knockout
- FGF
Fibroblast growth factor
- HIF
Hypoxia-inducible factor
- IGF
Insulin-like growth factor
- iEC-SynKO
Induced Endothelial Synectin KO
- MCP1
Monocyte Chemotactic Protein 1
- Micro-CT
Micro-Computed Tomography
- NPY
Neuropeptide Y
- PDGF
Platelet-derived growth factor
- PDGFR-β
Platelet-derived growth factor receptor βeta
- SMC
Smooth muscle cell
- SMKO
Smooth muscle cell conditional knockout
- Sm-MHC
Smooth muscle myosin heavy chain
- VEGF
Vascular endothelial cell growth factor
- VEGFR
Vascular endothelial cell growth factor receptor
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- 4.Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, Logan S, Yin L. Coronary collateral growth--back to the future. Journal of molecular and cellular cardiology. 2012;52:905–911. doi: 10.1016/j.yjmcc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 6.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circulation Research. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 7.Resnick N, Einav S, Chen-Konak L, Zilberman M, Yahav H, Shay-Salit A. Hemodynamic forces as a stimulus for arteriogenesis. Endothelium. 2003;10:197–206. doi: 10.1080/10623320390246289. [DOI] [PubMed] [Google Scholar]
- 8.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 9.Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. American journal of physiology. Heart and circulatory physiology. 2002;283:H2012–H2020. doi: 10.1152/ajpheart.00257.2002. [DOI] [PubMed] [Google Scholar]
- 10.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 11.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 2010;42:469–479. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nature reviews. Drug discovery. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 13.Simons M. Angiogenesis: Where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 14.Mac Gabhann F, Peirce SM. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation. 2010;17:333–347. doi: 10.1111/j.1549-8719.2010.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simons M. Chapter 14. Assessment of arteriogenesis. Methods in enzymology. 2008;445:331–342. doi: 10.1016/S0076-6879(08)03014-0. [DOI] [PubMed] [Google Scholar]
- 16.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of pdgf-bb and fgf-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 17.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-a specifies formation of native collaterals and regulates collateral growth in ischemia. Circulation Research. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Current opinion in hematology. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaper W. Collateral circulation: Past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circulation Research. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 22.Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. Modulation of collateral artery growth in a porcine hindlimb ligation model using mcp-1. American journal of physiology. Heart and circulatory physiology. 2003;284:H1422–H1428. doi: 10.1152/ajpheart.00506.2002. [DOI] [PubMed] [Google Scholar]
- 23.Robich MP, Matyal R, Chu LM, Feng J, Xu SH, Laham RJ, Hess PE, Bianchi C, Sellke FW. Effects of neuropeptide y on collateral development in a swine model of chronic myocardial ischemia. Journal of molecular and cellular cardiology. 2010;49:1022–1030. doi: 10.1016/j.yjmcc.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenza GL. Regulation of tissue perfusion in mammals by hypoxia-inducible factor 1. Exp Physiol. 2007;92:988–991. doi: 10.1113/expphysiol.2006.036343. [DOI] [PubMed] [Google Scholar]
- 25.Patel TH, Kimura H, Weiss CR, Semenza GL, Hofmann LV. Constitutively active hif-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovascular research. 2005;68:144–154. doi: 10.1016/j.cardiores.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC. Endothelial hif-2alpha regulates murine pathological angiogenesis and revascularization processes. The Journal of clinical investigation. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. Pr39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49–55. doi: 10.1038/71527. [DOI] [PubMed] [Google Scholar]
- 28.Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, Li W, Singh J, Simons M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 2005;112:2501–2509. doi: 10.1161/CIRCULATIONAHA.105.542829. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. Vegf receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. American journal of physiology. Heart and circulatory physiology. 2005;288:H759–H768. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- 30.Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 31.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Developmental cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. Vegf receptor 2 endocytic trafficking regulates arterial morphogenesis. Developmental cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, Toomre D, Ruhrberg C, Simons M. The neuropilin 1 cytoplasmic domain is required for vegf-a-dependent arteriogenesis. Developmental cell. 2013;25:156–168. doi: 10.1016/j.devcel.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: A psd-95/dlg/zo-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. The Journal of neuroscience : The official journal of the Society for Neuroscience. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedkov EI, Thomas MT, Sonka M, Yang F, Chittenden TW, Rhodes JM, Simons M, Ritman EL, Tomanek RJ. Synectin/syndecan-4 regulate coronary arteriolar growth during development. Developmental dynamics : An official publication of the American Association of Anatomists. 2007;236:2004–2010. doi: 10.1002/dvdy.21201. [DOI] [PubMed] [Google Scholar]
- 36.Paye JM, Phng LK, Lanahan AA, Gerhard H, Simons M. Synectin-dependent regulation of arterial maturation. Developmental dynamics : An official publication of the American Association of Anatomists. 2009;238:604–610. doi: 10.1002/dvdy.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of cre recombinase and egfp in transgenic mice. Physiol Genomics. 2002;10:211–215. doi: 10.1152/physiolgenomics.00054.2002. [DOI] [PubMed] [Google Scholar]
- 38.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: Impaired lymphocyte migration to bone marrow. The Journal of experimental medicine. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-b2 controls vegf-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 40.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 41.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 42.Jaba IM, Zhuang ZW, Li N, Jiang Y, Martin KA, Sinusas AJ, Papademetris X, Simons M, Sessa WC, Young LH, Tirziu D. No triggers rgs4 degradation to coordinate angiogenesis and cardiomyocyte growth. The Journal of clinical investigation. 2013;0:0–0. doi: 10.1172/JCI65112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey AM, O'Neill TJt, Morris CE, Peirce SM. Arteriolar remodeling following ischemic injury extends from capillary to large arteriole in the microcirculation. Microcirculation. 2008;15:389–404. doi: 10.1080/10739680701708436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. Nih image to imagej: 25 years of image analysis. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. [and] European Society for Dermatological Research. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 46.Peirce SM, Skalak TC. Microvascular remodeling: A complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y, Harrington A, Yang X, Friesel RE, Liaw L. The contribution of the tie2+ lineage to primitive and definitive hematopoietic cells. Genesis. 2010;48:563–567. doi: 10.1002/dvg.20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoefer IE, Grundmann S, van Royen N, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: Specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis. 2005;181:285–293. doi: 10.1016/j.atherosclerosis.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 49.Cochain C, Rodero MP, Vilar J, Récalde A, Richart AL, Loinard C, Zouggari Y, Guérin C, Duriez M, Combadière B, Poupel L, Lévy BI, Mallat Z, Combadière C, Silvestre J-S. Regulation of monocyte subset systemic levels by distinct chemokine receptors controls post-ischaemic neovascularization. Cardiovascular research. 2010;88:186–195. doi: 10.1093/cvr/cvq153. [DOI] [PubMed] [Google Scholar]
- 50.Eichmann A, Simons M. Vegf signaling inside vascular endothelial cells and beyond. Current opinion in cell biology. 2012;24:188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons M. An inside view: Vegf receptor trafficking and signaling. Physiology. 2012;27:213–222. doi: 10.1152/physiol.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the pdz domain of gipc/synectin recruits myosin vi to endocytic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Lanahan AA, Simons M. Vegfr2 trafficking: Speed doesn't kill. Cell cycle. 2013;12 doi: 10.4161/cc.25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai WJ, Kocsis E, Wu X, Rodriguez M, Luo X, Schaper W, Schaper J. Remodeling of the vascular tunica media is essential for development of collateral vessels in the canine heart. Mol Cell Biochem. 2004;264:201–210. doi: 10.1023/b:mcbi.0000044389.65590.57. [DOI] [PubMed] [Google Scholar]
- 55.Deng Y, Larrivee B, Zhuang ZW, Atri D, Moraes F, Prahst C, Eichmann A, Simons M. Endothelial raf1/erk activation regulates arterial morphogenesis. Blood. 2013;121:3988–3996. S3981–S3989. doi: 10.1182/blood-2012-12-474601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of pdgf-b and pdgfr-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 57.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial pdgf-b retention is required for proper investment of pericytes in the microvessel wall. Genes & development. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren B, Deng Y, Mukhopadhyay A, Lanahan AA, Zhuang ZW, Moodie KL, Mulligan-Kehoe MJ, Byzova TV, Peterson RT, Simons M. Erk1/2-akt1 crosstalk regulates arteriogenesis in mice and zebrafish. The Journal of clinical investigation. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. Journal of the American College of Cardiology. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Waltenberger J. Vegf resistance as a molecular basis to explain the angiogenesis paradox in diabetes mellitus. Biochemical Society transactions. 2009;37:1167–1170. doi: 10.1042/BST0371167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.