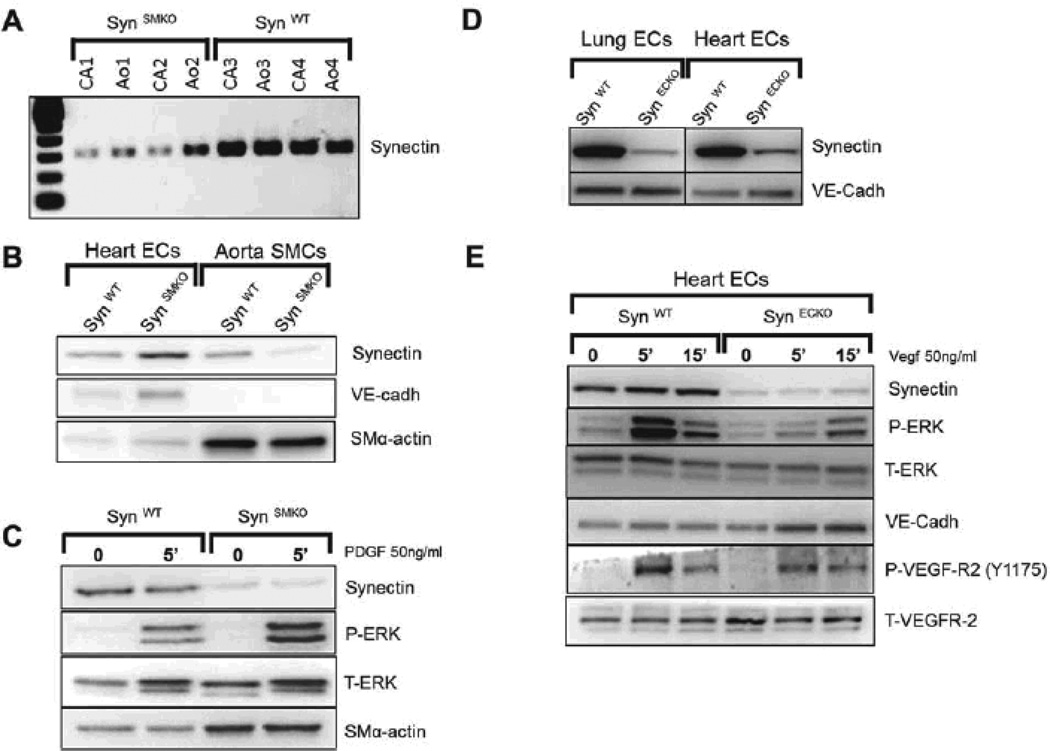
Figure 2. Validation of SynSMKO and SynECKO mouse lines.
(A–C) In vivo ablation of synectin expression in smooth muscle cells (SynSMKO). (A) Decreased synectin RNA levels in carotid (CA) and aorta (Ao) from SynSMKO mice compared with control littermates. (B) Western blot of total cell lysates of aortic smooth muscle (SMC) and heart endothelial cells (EC) from SynSMKO mice. Note a reduction in synectin protein levels in SMC but not in EC. (C) ERK activation: Western blot analysis of total cell lysate of aortic SMC. Confluent, serum starved cells were stimulated for 5 min with 50ng/ml PDGF-BB. Phosphorylation of ERK (P-ERK) in response to PDGF-BB is not altered in synectin null smooth muscle cells (SynSMKO) relative to wild type smooth muscle cells (SynWT). T-ERK: total ERK. SM-α-actin: SMC α-actin. (D, E) In vivo ablation of synectin expression in endothelial cells (SynECKO).(D) Decreased synectin protein levels in endothelial cells isolated from SynECKO mice compared with SynWT mice. (E) Western blotting of total cell lysates isolated from heart endothelial cells (EC). Confluent, serum starved EC were stimulated for the times indicated with 50 ng/ml VEGF-A165. Note reduced phosphorylation of ERK (P-ERK) and in VEGFR2 Y1175 site in synectin null endothelial cells (SynECKO).