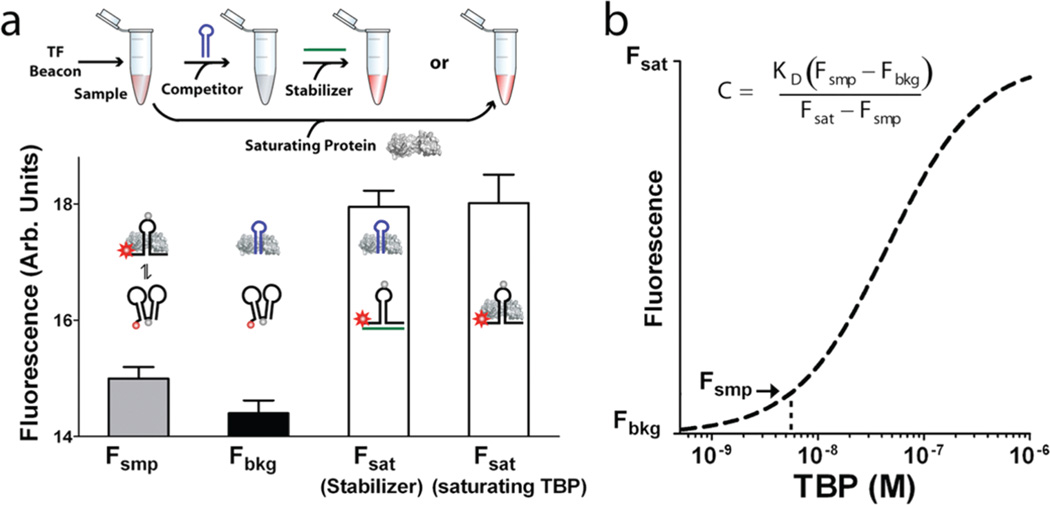
Figure 3.
TF beacons support the quantification of DNA-binding activity directly in crude nuclear extracts. (a) This requires three fluorescence measurements, which are performed in a single tube: Fsmp, the fluorescence of the TF beacon in equilibrium with the endogenous target in the sample; Fbkg, the fluorescence of the TF beacon when unbound, obtained by adding a saturating concentration of a competitor DNA (blue); and Fsat, the fluorescence of the TF beacon in its fully bound state, which is obtained by adding either a DNA “stabilizer” (green) or excess target, each of which stabilize the beacon’s emissive conformation. (b) From the known dissociation constant of the beacon, we then estimate the target concentration. For active TBP, we measure a concentration of 5.8 ± 1.6 nM (or 5.7 ± 1.7 nM if Fsat is obtained using saturating TBP) in HeLa nuclear extract, which is within error of the value obtained using a traditional gel shift assay (Supporting Methods and Supporting Figure 4).