Figure 3.
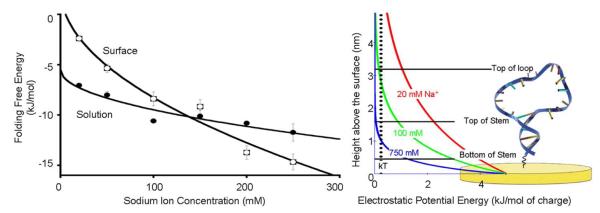
(left) At low ionic strength our DNA stem-loop is less stable on the surface than in solution, presumably due to electrostaticr epulsion from the surface, which is negatively charged at the potential we have employed. At higher ionic strength electrostatic screening increases, negating this effect. Under these conditions the excluded volume effect dominates and the surface-attached molecule becomes more stable than the equivalent molecule in solution. (right) This behavior occurs because the electric field near a charged surface in an electrolyte is a strong function of counterion concentration. At higher sodium ion concentrations the electric field falls to near zero over distances shorter than a single base pair in double-stranded DNA. At lower ionic strengths, in contrast, the electric field remains significant over distances comparable to the size of our DNA stem-loop. The folding free energies observed in solution exhibit the expected40square root dependence on ionic strength. We fitted the surface energies to a square root dependence; this is not theoretically justified but serves as a convenient guide to the eye. The error bars in this and the following plots are standard errors derived from replicate, independent measurements.
