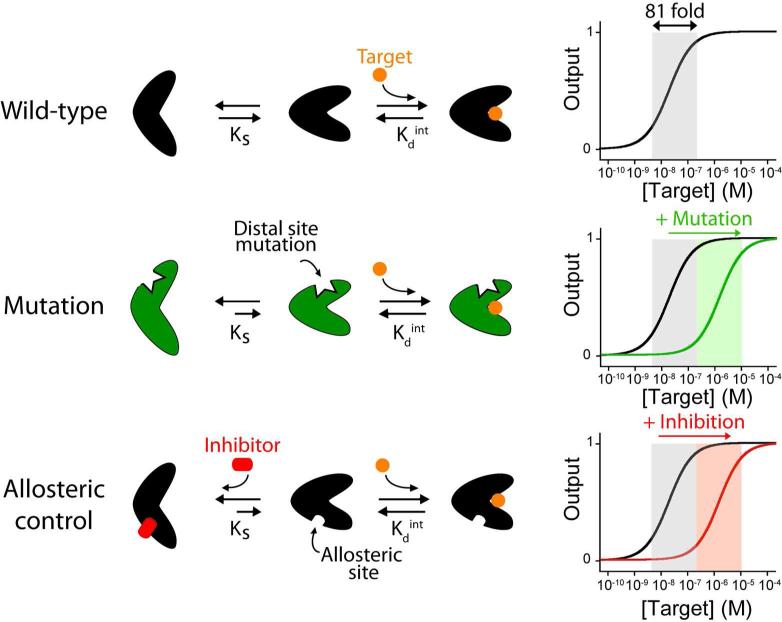
Figure 1.
Schematic representations of some of the strategies used by nature to tune the affinity of her receptors. (Top) For many receptors target binding shifts a pre-existing equilibrium between a binding competent state and a non-binding state10. The affinity of the receptor for its target is a function of both the intrinsic affinity of the binding-competent state (Kdint) and the switching equilibrium constant, Ks. (Middle) Mutations at the distal site of the receptor can stabilize the non-binding state thus shifting the dynamic range towards higher target concentrations. (Bottom) The binding of an allosteric inhibitor can also be used to stabilize the non-binding state, reducing Ks and thus raising the overall dissociation constant.