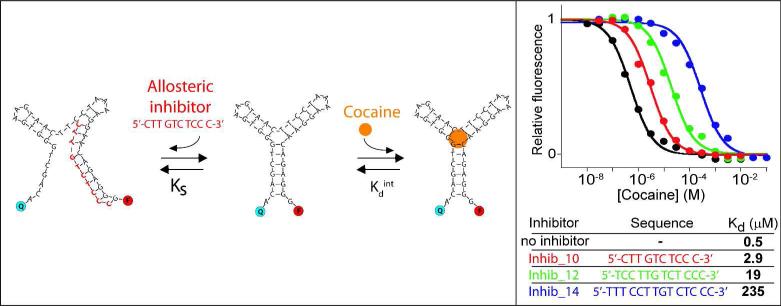
Figure 3.
The affinity of the cocaine-binding aptamer can be efficiently and precisely tuned using allosteric inhibition. To do this we designed oligonucleotides complementary to a sequence within the parent aptamer such that hybridization with the inhibitor competes with folding of the aptamer into its binding-competent conformation. By varying the length and/or concentration (and thus hybridization energy) of the inhibitor we can then tune the affinity of the aptamer over many orders of magnitude. As expected for single-site binding the useful dynamic range of the inhibited aptamer still spans the classic 81-fold of target concentration as the presence of the inhibitor only affects the switching equilibrium thermodynamics and not the signalling mechanism. Here the inhibitor concentration was held at 100 nM concentration except for shortest inhibitor (inhib_10) which was set at 300 nM (see also Figure S3).