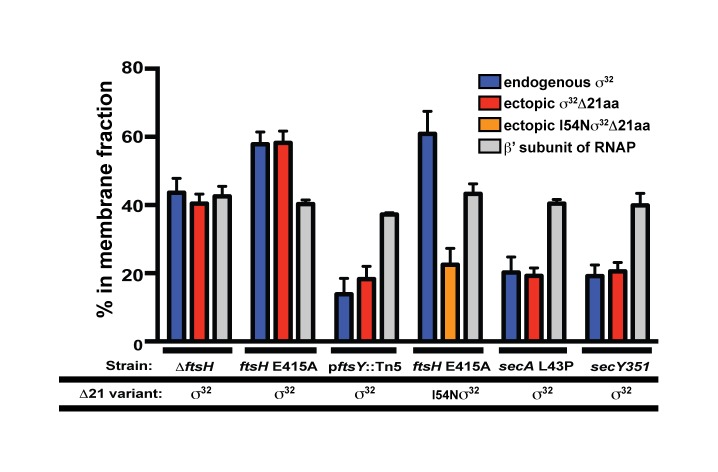
Figure 5. σ32 is partially membrane associated.
The extent of association of σ32 and the β′ subunit of RNA polymerase with the membrane fraction was determined by quantitative immunoblotting of the soluble and nonsoluble fractions. Membrane association of σ32 and β′ was assessed in several relevant strain backgrounds. In addition to endogenous σ32, all strains contained a plasmid-encoded variant of σ32 lacking its 21 C-terminal amino acids (σ32Δ21aa). Ectopically expressed σ32Δ21aa or I54Nσ32Δ21aa were present at levels comparable to native σ32 and were distinguished from endogenous σ32 on a 12% SDS-PAGE gel. All fractionation experiments were performed ≥8 times, and % fractionation was calculated from experiments where probed cytoplasmic (RuvB) and membrane (RseA) proteins separated properly.