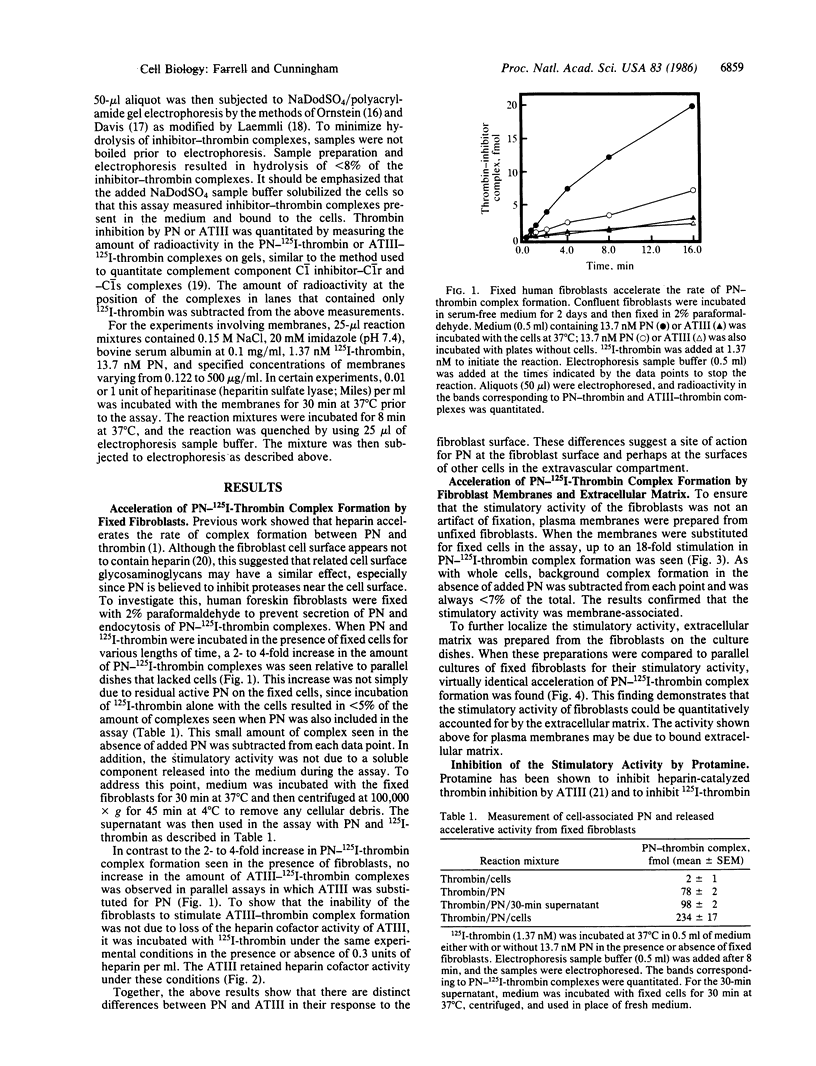
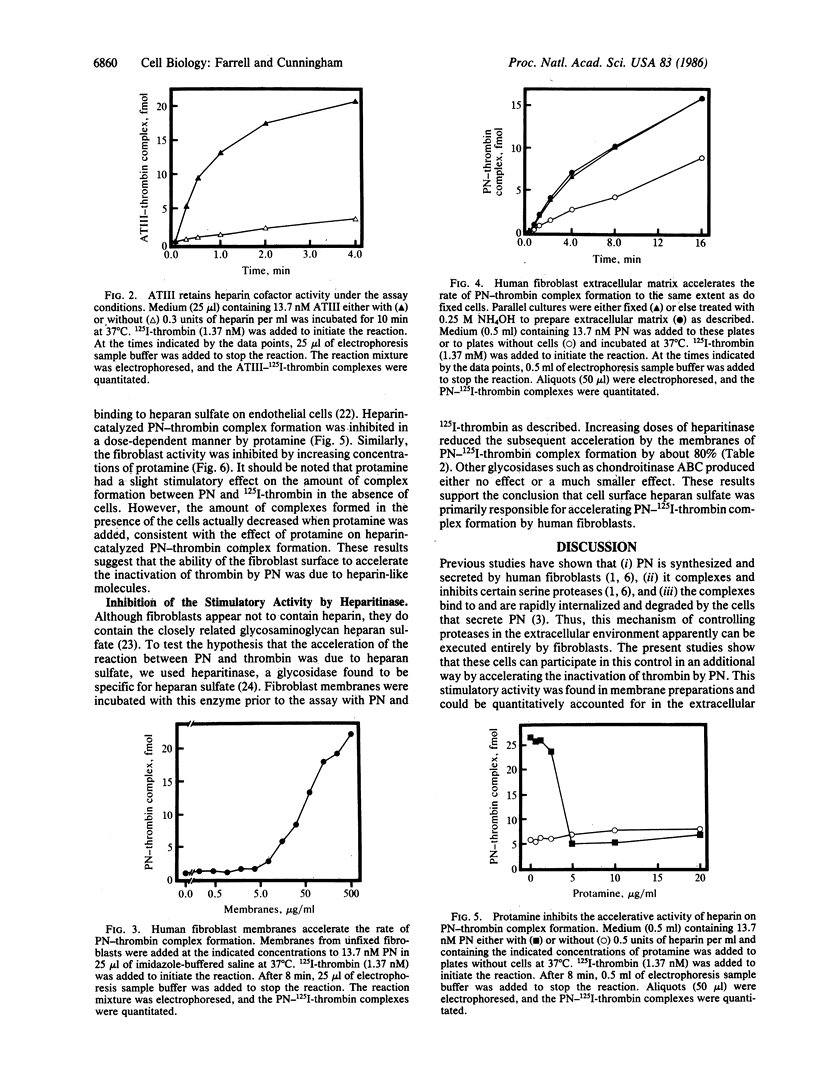
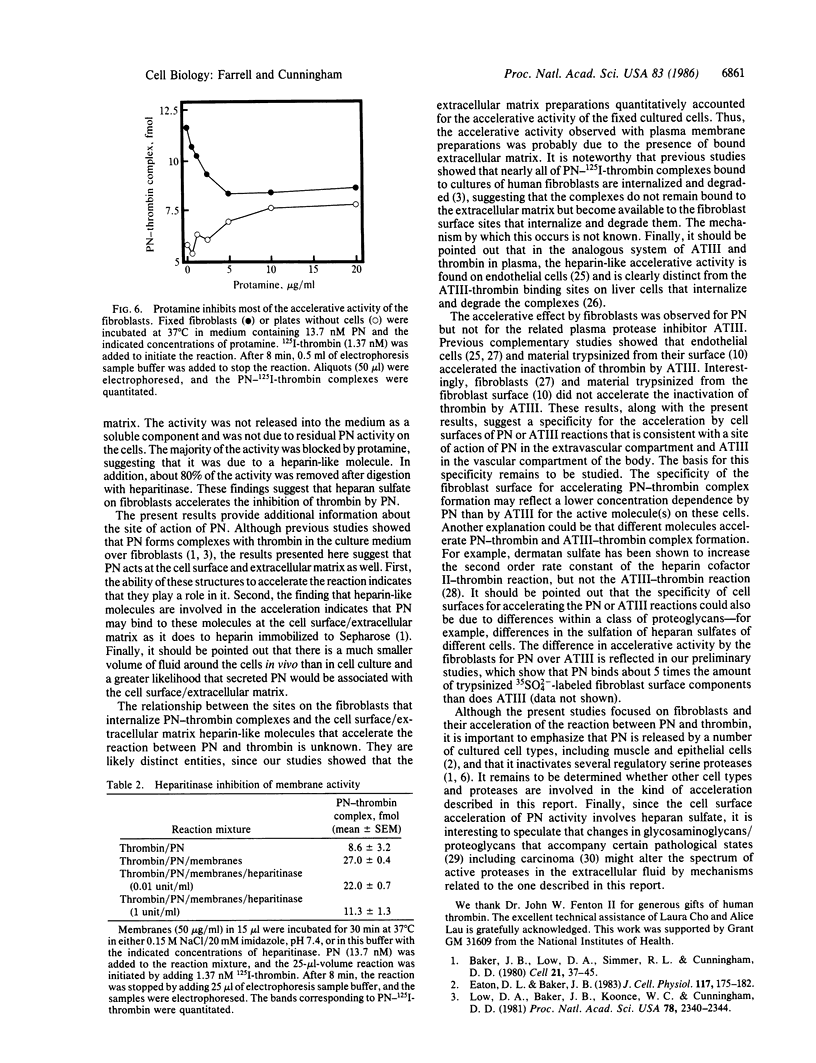
Abstract
Protease nexin (PN) is a protein protease inhibitor secreted by human fibroblasts in culture that complexes and inhibits certain regulatory serine proteases. The PN-protease complexes then bind to these cells and are rapidly internalized and degraded. This report shows that the fibroblast surface accelerates the formation of PN-thrombin complexes. In contrast, it did not accelerate the formation of complexes between thrombin and antithrombin III, a closely related protease inhibitor found in plasma. These results support a role for PN in the regulation of certain proteases in the extravascular compartment at and near the surface of tissue cells. The activity that accelerated PN-thrombin complex formation was membrane-associated, since fixed cells, purified membranes, and extracellular matrix preparations all contained this activity. The ability of cells to accelerate the reaction between PN and thrombin was inhibited by protamine, suggesting that the activity was similar to that of heparin. Heparitinase digestion of plasma membranes prior to assay reduced the activity by about 80%, suggesting that heparan sulfate may account for most of the accelerative activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Bergman B. L., Scott R. W., Bajpai A., Watts S., Baker J. B. Inhibition of tumor-cell-mediated extracellular matrix destruction by a fibroblast proteinase inhibitor, protease nexin I. Proc Natl Acad Sci U S A. 1986 Feb;83(4):996–1000. doi: 10.1073/pnas.83.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Baker J. B. Evidence that a variety of cultured cells secrete protease nexin and produce a distinct cytoplasmic serine protease-binding factor. J Cell Physiol. 1983 Nov;117(2):175–182. doi: 10.1002/jcp.1041170207. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fuchs H. E., Shifman M. A., Michalopoulos G., Pizzo S. V. Hepatocyte receptors for antithrombin III-proteinase complexes. J Cell Biochem. 1984;24(3):197–206. doi: 10.1002/jcb.240240302. [DOI] [PubMed] [Google Scholar]
- Guenther J., Nick H., Monard D. A glia-derived neurite-promoting factor with protease inhibitory activity. EMBO J. 1985 Aug;4(8):1963–1966. doi: 10.1002/j.1460-2075.1985.tb03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Kitani T., Nagarajan S. C., Shanberge J. N. Effect of protamine on heparin-antithrombin III complexes. In vitro studies. Thromb Res. 1980 Feb 1;17(3-4):367–374. doi: 10.1016/0049-3848(80)90071-7. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Silbert J. E., Silbert C. K. Heparan sulfate of skin fibroblasts grown in culture. Connect Tissue Res. 1975;4(1):17–23. doi: 10.3109/03008207509152193. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Scott R. W., Baker J. B., Cunningham D. D. Cells regulate their mitogenic response to thrombin through release of protease nexin. Nature. 1982 Jul 29;298(5873):476–478. doi: 10.1038/298476a0. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Fritze L., Galli S. J., Karp G., Rosenberg R. D. Microvascular heparin-like species with anticoagulant activity. Am J Physiol. 1983 Nov;245(5 Pt 1):H725–H733. doi: 10.1152/ajpheart.1983.245.5.H725. [DOI] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Shimada K., Ozawa T. Evidence that cell surface heparan sulfate is involved in the high affinity thrombin binding to cultured porcine aortic endothelial cells. J Clin Invest. 1985 Apr;75(4):1308–1316. doi: 10.1172/JCI111831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Arlaud G. J., Colomb M. G. Kinetics of reaction of human C1-inhibitor with the human complement system proteases C1r and C1s. Biochim Biophys Acta. 1980 Apr 11;612(2):433–449. doi: 10.1016/0005-2744(80)90126-6. [DOI] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tollefsen D. M., Pestka C. A., Monafo W. J. Activation of heparin cofactor II by dermatan sulfate. J Biol Chem. 1983 Jun 10;258(11):6713–6716. [PubMed] [Google Scholar]
- Wight T. N. Proteoglycans in pathological conditions: atherosclerosis. Fed Proc. 1985 Feb;44(2):381–385. [PubMed] [Google Scholar]


