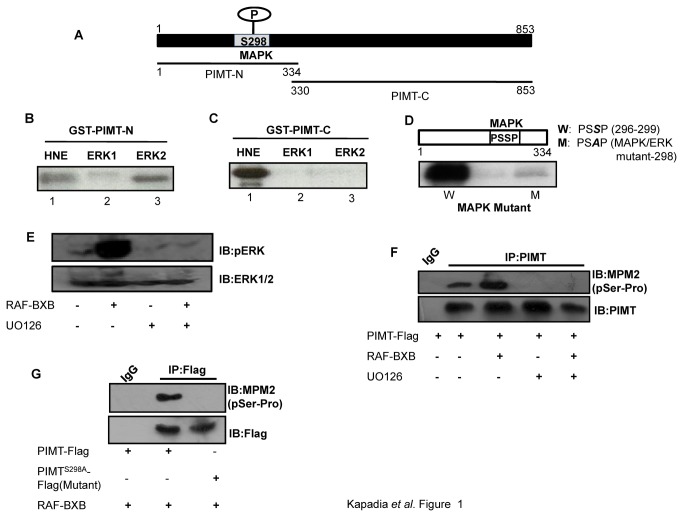
Figure 1. PIMT is a substrate of MAPK.
(A) Schematic diagram of PIMT with a potential phosphorylation site of ERK1/2 at Ser298. PIMT protein was fragmented into 2 parts, PIMT-N (1-334) and PIMT-C (330-853) and fused to GST. (B&C) GST-PIMT-N (B) and GST-PIMT-C (C) bound to glutathione sepharose beads were subjected to kinase reaction in the presence of HeLa nuclear extract [HNE] and constitutively active purified MAPKs ERK1 and ERK2. (D) Glutathione sepharose beads bound GST-PIMT-N [W] and GST-PIMTS298A [M] were subjected to kinase assay with active and purified ERK2. Mutation at MAPK recognition site [PSSP] abolished phosphorylation of PIMT.(E)293T cells were transfected with vector or RAF-BXB and 24 h post transfection cells were treated with DMSO or 10 µM UO126 for 30 min. Subsequently cells were lysed and resolved on 10% SDS-PAGE and probed with Anti-pERK1/2. Blots were stripped and reprobed withAnti-ERK1/2. (F & G) 293T cells were transfected with pCMV-PIMT Flag (F, G) or pCMV- PIMTS298A Flag (G) along with RAF-BXB (F, G) and cells were treated with UO126 where indicated (F). Post transfection cells were cultured in DMEM containing 1% FBS overnight, PIMT was immunoprecipitated with Anti-PIMT followed by separation on 10% SDS-PAGE and probed with Anti-MPM2 (F, G). Blots were stripped and reprobed with Anti-PIMT (F) or Anti-Flag (G) as mentioned.