Abstract
Claudin-20 is a member of the Claudin family of transmembrane proteins located in the tight junction (TJ) of cells of epithelial origin. Due to the increasing evidence supporting the role of TJ proteins in preventing tumor cell metastatic behavior, this study sought to evaluate the distribution of Claudin-20 in human breast cancer and the effect of Claudin-20 overexpression in human breast cancer cells.
Q-PCR data from breast cancer primary tumors (n = 114) and matched background tissue (n = 30) showed that high claudin-20 expression was correlated with poor survival of patients with breast cancer (p = 0.022). Following transformation of the breast cancer cell lines MDA-MB-231 and MCF7 with a Claudin-20 expression construct functional assays were performed to ascertain changes in cell behavior. Claudin-20 transformed cells showed significantly increased invasion (p < 0.005) and were significantly less adhesive than wild type cells (p < 0.05). There was no effect on growth (either in vitro or in vivo) for either cell line. Overexpression of Claudin-20 resulted in reduced transepithelial resistance (induced by the motogen HGF at 25 ng/ml, p = 0.0007). Interestingly, this was not mirrored by paracellular permeability, as overexpression of Claudin-20 caused a decrease in permeability.
The introduction of Claudin-20 into human breast cancer cells resulted in breast cancer cells with an aggressive phenotype and reduced trans-epithelial resistance. There was no corresponding decrease in paracellular permeability, indicating that this Claudin has a differential function in epithelial TJ. This provides further insight into the importance of correctly functioning TJ in preventing the progression of human breast cancer.
Keywords: Claudin-20, breast cancer, metastasis, survival, tight junction
Introduction
For progression of cancer metastases it is essential for cancer cells to dissociate from the primary tumor and penetrate the vascular endothelium. For dissociation to occur there must be a loss in cell-cell adhesion and, in epithelial cells, these cell to cell associations are composed three distinct but interacting components, the tight junctions (TJs) which are usually situated at the apex of the lumen-facing membrane, the adherens junctions which are the next structure within the junction structures and the desmosomes.
Due to their position, TJs consequently provide an essential barrier for cancer cells to overcome in order to metastasise. TJs provide a barrier that is able to selectively regulate diffusion of a number of substances (small molecules, water, ions) through the between adjoining cells and thus are regulators of paracellular permeability.1 They also support the maintenance of cell polarity by functioning as a molecular fence, thus restricting the diffusion of basolateral and apical membrane structures.2 Importantly, it has been shown that TJs of vascular endothelium can act in vivo as a barricade against metastatic cancer cells.3
The protein components of TJs can be divided into the transmembrane proteins which include the TJ-associated MARVEL Proteins (TAMP) (occludin, tricellulin, MARVELD3), (J)unctional (A)dhesion (M)olecules and the Claudins; the cytoplasmic plaque/anchoring proteins include Zona Occludens -1, -2, -3 (ZO-1, ZO-2, ZO-3); and associated regulatory proteins including α-catenin, cingulin etc. The trans-membrane proteins are linked to the cytoplasmic anchoring proteins via scaffolding and adaptor proteins, together with signaling proteins and linkers to the cytoskeletal. The ZO family and other PDZ proteins are bound to the cytoplasmic tails of the trans-membrane proteins.4 Studies have indicated that components of the TJ complex are involved directly or indirectly during the metastasis of breast cancer.5-9 One such family of TJ proteins is the Claudin family of transmembrane proteins which was originally identified by Furuse et al.10 who described Claudin-1 and -2. Claudins have a role as regulators of paracellular selectively, however, new roles for Claudins have been proposed showing that they are involved in cell growth and in (E)pithelial-(M)esenchymal (T)ransition, not just as cell adhesion proteins.10
To date, over 20 members have been described which can be divided into the so called “classic Claudins,” which include members with high sequence homology including Claudin-1 to -10, -14,-15, -17 and -19, and the “non-classic” Claudins which include Claudin-11, -13, -16, -18, and -20 to -24.11 Studies have shown that Claudin family members vary in expression depending on location and cell type, with members of this proein family having a PDZ domain in their COOH- terminal allowing interaction with the TJ cytoplasmic proteins such as ZO-1, linking the Claudins to the actin cytoskeleton.12 Cytoskeletal changes, as well as changes to cell to cell adhesion and extracellular matrix changes, are needed for cancer cells to become more motile in order to metastasise, thus Claudins would seem to have a role to play in this progression.
A Increasingly, the importance of the Claudins in cancer progression has been demonstrated, with a reduction in Claudin-16 being linked to aggressive tumors and high mortality in human breast cancer patients.13 Similarly, overexpression of Claudin-5 renders HECV (human endothelial cells from vein) to be less motile and less adhesive cells.14 In skin papilloma, Claudin-1, -6, -11, -12 and -18 have been shown to be downregulated, with Claudin-2, -3 and -4 being upregulated in intestinal carcinoma.15-17 Downregulation of Claudin-1, -6 and -7 has been shown in esophageal squamous cell carcinoma with Claudin-1, -2 and -12 upregulation and Claudin-7 and -8 downregulation seen in colorectal carcinoma.18-25
In breast cancer, Claudins-1 and -4 have been shown to have significantly higher expression in cancers that display basal-like subtype characteristics (Her-2−ve, ER−ve, CK5/6+ve, EGFR+ve). It has been suggested that when Claudins -1 and -4 expression is increased it is associated with this basal-like breast cancer subtype of, which is often related to poorer outcomes.26 Moreover, increases in Claudin-4 linked to adverse outcome including patients that have received adjuvant tamoxifen.27
There is little clear evidence supporting a role for Claudin-20 which was originally described as being abundantly distributed in squamous-differentiated bronchial epithelial cells by Chen et al.28 Also, Claudin-20 expression has been shown to increase in retinal pigment epithelium during late development of TJs in chick embryos.29 Due to the increasing evidence supporting the role of TJ proteins in preventing tumor cell metastatic behavior, this study sought to evaluate the how overexpression of Claudin-20 might affect the behavior of human breast cancer cells.
Results
Quantitative PCR analysis of Claudin-20 in human breast tissues and prognostic indicators
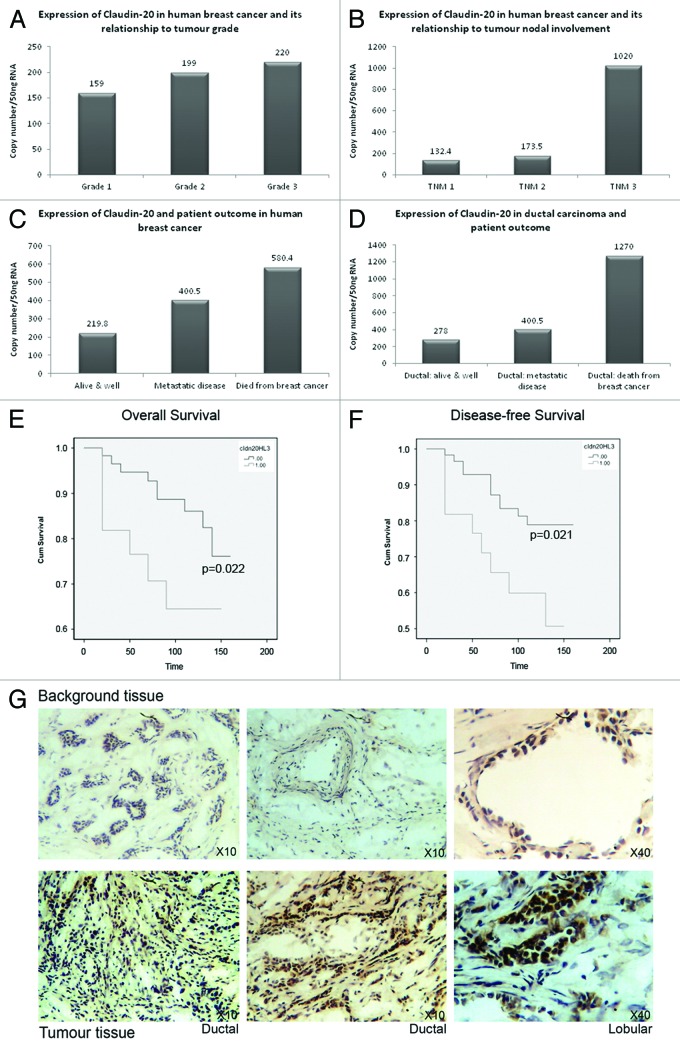
Quantitative-PCR analysis of gene transcripts levels (normalized using CK-19/GAPDH) was used to assess differences in expression in human breast tissues of Claudin-20. We compared the expression of Claudin-20 with the diagnostic indicators NPI (Nottingham Prognostic Indicator), Grade and TNM status (Tumor nodal involvement: This characterizes the staging of tumors by evaluating (T) primary tumor, (N) nodal involvement (M) metastasic disease presence). Node positive tumors exhibited increased Claudin-20 in comparison to node negative tumors (2874+/−1128, median 1324 vs. 4548+/−1549, median 2780, respectively p = 0.07). There was also increasing expression of Claudin-20 associated with higher tumor grade, which did not reach significance (Fig. 1A) and TNM status, p = 0.058 (Fig. 1B). There was no association of Claudin-20 with NPI (Nottingham Prognostic Indicator) status.
Figure 1. Expression of Clauin-20 in normal and cancerous human breast tissue. Increased expression of Claudin-20 with tumor grade (A). Claudin-20 and tumor nodal involvement (B). Patient outcome and increased levels of Claudin-20 (C). Relationship of Claudin-20 to patient outcome in ductal carcinoma (D). Overall (E) and disease-free (F) survival of patients with breast cancer and expression of Claudin-20 (Claudin-20 “Low” vs. “high”). Representative sections of background (top) and tumor (bottom) tissue after IHC to demonstrate increased levels of Claudin-20 protein in breast cancer (G).
Claudin-20 expression and patient clinical outcome
Median values showed that Claudin-20 expression was related to patient outcome in those with metastatic disease and in those where breast cancer had caused death (Fig. 1C). When examining survival of patients with ductal carcinoma, Claudin-20 was elevated in those whose breast cancer had resulted in death from the disease (Fig. 1D) (those patients with ductal cancer who remained alive and well: 3828+/−1180, median 278 vs. patients with ductal cancer who died from the disease: 9978+/− 6321, median 849), although this was not significant.
Claudin-20 values were also increased metastatic disease and death from breast cancer and in those who had bone metastasis (5347+/− 2827, median: 400 and 8231+/− 6102, median 3327 respectively vs. alive and well 3327+/− 971, median 219.8).
Claudin-20 and patient survival
Kaplan-Meier curves were calculated to assess survival (Fig. 1E and F). Higher levels of Claudin-20 transcript were related to reduced shorter overall survival when compared with patients with low levels of Claudin-20 (p = 0.022); high mean survival 106.594 mo (83.823–129.753 mo, 95% CI) vs. low mean survival 140.381months (130.65–150.111 mo, 95% CI (see Martin et al. 2008 for cut-offs)). A significantly shorter disease-free survival was associated with high levels of Claudin-20 (p = 0.021); high mean survival 100.444 mo (77.317–123.571 mo, 95% CI) vs. low mean survival 135.923months (124.959–146.888 mo, 95% CI).
Immunohistochemical staining of Claudin-20 in human breast tissues
Representative sections of tumor and normal human breast tissue sections are shown in Figure 1G. Claudin-20 was observed to stain more heavily in tumor tissues (bottom panel), when compared with background breast tissue (top panel). This supported the data from transcript level analysis. The less than typical staining pattern may be due to a difference in TJ of cancer cells- they often do not assemble correctly due to aberrant expression of TJ proteins, i.e., either increased or reduced levels. Moreover, the function of Claudin-20 in breast cells may not be the typical fence/barrier function.
Overexpression of Claudin-20 in cancer cell lines derived from human breast cancer
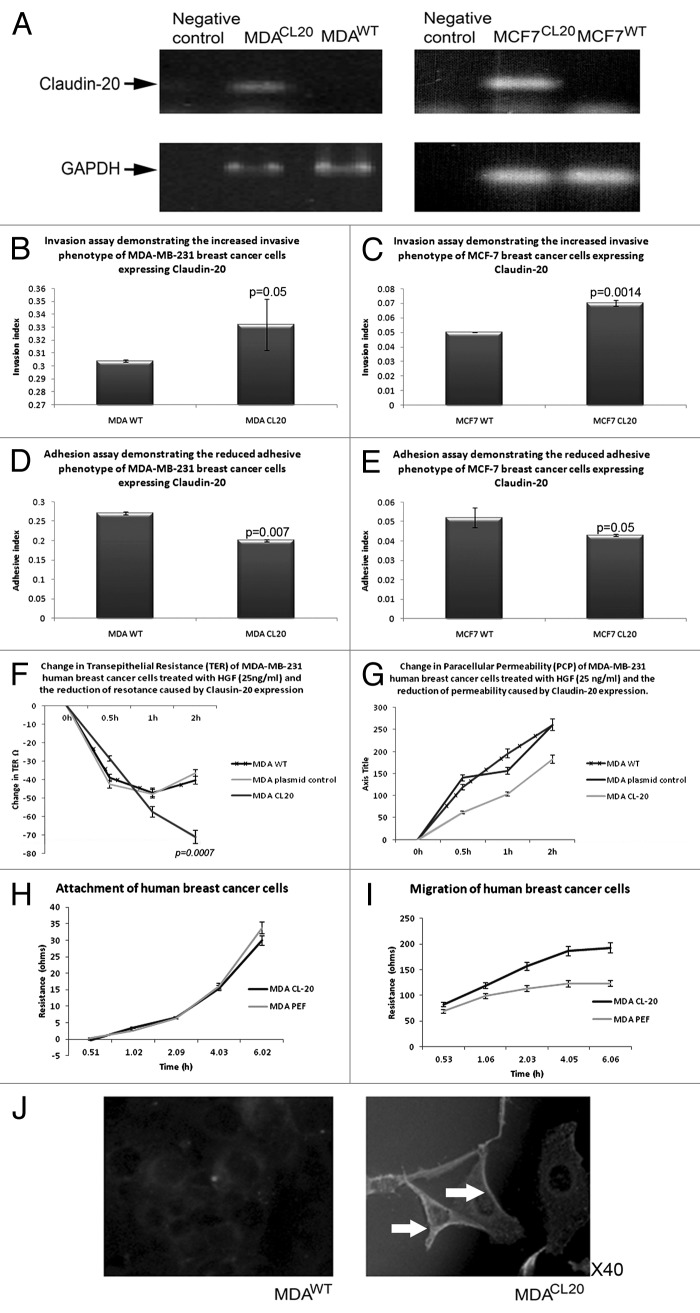
RT-PCR revealed that MDA-MB-231 and MCF7 breast cancer cells have very low levels of Claudin-20; we therefor determined to overexpress Claudin-20 in these cells in order to investigate the role of Claudin-20 in breast cancer. Claudin-20 was successfully overexpressed in both cell lines (MDACL20/MCF7CL20), Figure 2A. A number of in vitro function assays were then performed to assess changes in cell behavior. There was no observed difference in cellular morphology of the Claudin-20 overexpression cells.
Figure 2. Effect of CLaudin-20 overexpression in human breast cancer cells. Confirmation of CLaudin-20 expression in MDA-MB-231(left) and MCF7 (right) cells (A). Increased invasive behavior of MDA-MB-231 (B) and MCF7 (C) cells overexpressing Claudin-20. Reduced adhesion to basement membrane of MDA-MB-231 (D) and MCF7 (E) cells overexpressing Claudin-20. Clausin-20 overexpression reduces transepithelial resistance (F) and paracellular (G) of MDA-MB-231 cells. Claudin-20 did not change attachment of MDA-MB-231 cells (H). MDA-MB-231 cells overexpressing Claudin-20 exhibited increased migratory phenotype (I). Imunofluorescent staining to show confirmation of Claudin-20 overexpression in MDA-MB-231 cells (J).
Cellular behavior and Claudin-20 overexpression
Overexpression of Claudin-20 produced cells with a significantly increased invasive potential in both human breast cancer cell lines (MDACL-20 0.332 ± 0.02 RU, MDAWT 0.304 ± 0.001; p = 0.05: MCF-7CL-20 0.07 ± 0.002 RU, MCF-7WT 0.05 ± 0.0001; p = 0.0014) (Fig. 2B and 2C). Both MDACL-20 and MCF-7CL-20 cells were significantly less adhesive to basement membrane that were the wild type control cells (number of cells adhering to matrix: MDACL-20 0.2 ± 0.02 RU vs. MDAWT 0.27 ± 0.004, p = 0.007; MCF-7CL-20 0.043 ± 0.0005 RU vs. MCF-7WT 0.052 ± 0.0.005, p = 0.05) (Fig. 2D and 2E). When assessing tight junction functions, it was found that overexpression of Claudin-20 resulted in reduced TER in MDA-MB-231 cells (induced by the motogen HGF at 25 ng/ml: MDACL-20 change in TER at 2h -74+/−1.5 vs MDAWT at 2h -40+/−4.4, p = 0.0007), Figure 2F. Interestingly, this was not mirrored by changes in PCP, as overexpression of Claudin-20 caused a reduction in permeability (MDACL-20 change in PCP at 2h 182+/−5 vs MDAWT at 2h 260+/−10, p = 0.05), Figure 2G. Assessment of cellular attachment (via ECIS technology) demonstrated no difference after Claudin-20 expression (Fig. 2H), however, overexpression of Claudin-20 caused cells to be able to migrate at an increased rate, compared with the migration capabilities of wild type cells (Fig. 2I). There was no effect on growth rate in vitro for either MDA-MB-231 or MCF7 cell lines. Moreover, no significant difference in in vivo tumor growth rate for MDA-MB-231 cells was observed (tumor volume mm3: MDAWT 390.722+/−405.7 vs. MDACL-20 334.097+/−185.69, p = 0.59 at day 29).
Possible interactions for Claudin-20
As there is so little information on Claudin-20, we looked at possible interactions between Claudin-20 and other well know TJ proteins. Immunoprecipitation studies revealed possible interactions between all 3 ZO proteins (Fig. 3). Indeed, the strongest signal was for ZO-1 (H-300) rather than the C-terminal. There were also signals for three other Claudins (Claudin-11, -10 and -5) and the TJ transmembrane proteins JAM-B (VE-JAM), Nectin-3 and Occludin.
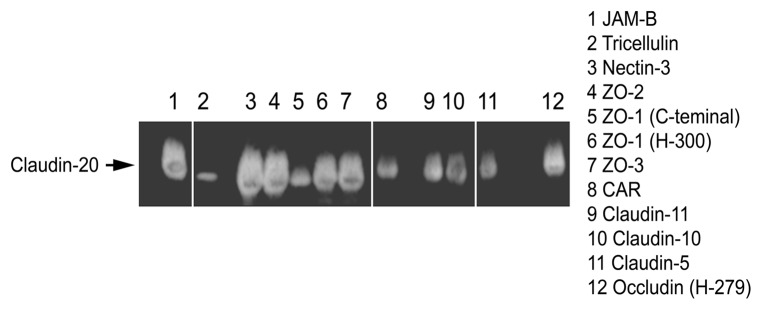
Figure 3. Immunoprecipitation of some tight junction proteins by Claudin-20.
Discussion
The expression of Claudin-20 in human breast cancer was found to be increased and was related to poor outcome and survival rates in our patient cohort. Moreover, overexpression of Claudin-20 promoted aggressive behavior in the two breast cancer cell lines (MDA-MB-231 and MCF-7). In recent years it has become apparent that members of the Claudin protein family have diverse expression profiles and functions beyond those initially assigned to them.
Claudin-20, also known as EMP-1 (epithelial membrane protein-1) and TMP (tumor-associated membrane protein) was first described by Chen et al.28 The authors described a gene that was novel and encoded a protein distinct from the peripheral myelin protein PMP22 but to which it was structurally related. EMP-1 mRNA was abundant in squamous-differentiated-bronchial-epithelial cells. Only low levels of were detected in other human tissues.29 EMP-1 was subsequently assigned to the Claudin protein superfamily as Claudin-20.30 Subsequent work on Claudin-20 has been sparse, however, in 2005, Jain et al.31 suggested that Claudin-20, or EMP-1 to be a surface biomarker, which might be correlated with the acquisition of resistance to the (E)pidermal (G)rowth (F)actor (R)eceptor (EGFR)/HER2 inhibitor, Gefitinib and was further correlated with the absence of response in patient lung cancer samples to Gefinitab as well as to secondary Gefitinib resistance and clinical progression. Acquisition of Gefitinib clinical resistance and Claudin-20 (or EMP-1) expression was independent of somatic mutations via Gefitinib-sensitizing EGFR, suggesting that Claudin-20 could be considered a biomarker for clinical resistance to Gefitinib. In addition, the authors suggested that the may be cross-talk between EGFR signaling pathway and Claudin-20.31 Whether or not this is the case in breast cancer remains to be seen.
However, a later study investigated the effect of Gefinitab on Bam1a breast tumor cells overexpressing HER2/neu and ErbB-3 in vitro and in vivo.32 In breast and other cancers, the HER2/neu oncogene is an important therapeutic target and a diagnostic and prognostic factor. Phosphorylation of the ErbB-3-EGFR-HER2/neu was reduced with Gefinitab treatment as was the phosphorylation of the signal transducers/activators of transcription. This happened in a dose-dependent fashion, leading to significant changes in cell behavior such as proliferation, which was linked to signaling via Akt. Oral administration of gefitinib in in vivo models, prevented outgrowth of Bam1a tumor cells, shrank established tumors, eliminated HER2/HER3 phosphorylation, decreased Akt and MAPK signaling. IR-5, a variant of Bam1a cells, bears a novel HER2/neu point mutation which correlates with a decrease to sensitivity to gefitinib showed increased expression of Claudin-20.32
In the current study, increased expression of Claudin-20 caused an increased invasive and motile phenotype. TJ function in the form of trans-epithelial resistance was reduced; however, permeability was not altered. Moreover, growth was not affected, in either in vitro or in vivo experiments. In TJ studies, trans-epithelial resistance and permeability measurements do not completely reflect changes in paracellular pathways: they are, all together however, composite for both trans-cellular and paracellular paths. TJs provide a barrier block paracellular leakage and trans-cellular transport is mediated by proteins situated both apically and basolaterally. The mechanism by which paracellular transport is regulated is little understood. Moreover, the maintenance of epithelial cell polarized architecture is an essential function of TJs. Claudins are involved in regulating the permeability of specific ions in a selective manner. This suggests that they may regulate and maintain paracellular ionic transfer, which can be evaluated in vitro using TER.33
In our study, overexpression of Claudin-20 resulted in decreased TER, which would tie in with an increase in aggressive behavior, namely increased invasion and motility of the breast cancer cells. However, there was some decrease in PCP, which may not have been anticipated. A disparate result between TER and PCP in Claudin upregulated cells is not unknown. It has been shown that overexpression of Claudin-5 in (M)adin-(D)arby-(C)anine-(K)idney II cells, resulted in an increase in trans-epithelial resistance and reduced monovalent cation conductance.31 However, the flux of charged/neutral monosaccharides via the paracellular pathway was not significantly changed; the authors concluded that permeability to ions was selectively reduced upon Claudin-5 expression. When Claudin-5 underwent site-directed mutation (first extracellular domain amino acid residues), the TJs formed exhibited different properties in response to Claudin-5 expression. Cysteine residues that are conserved were found to be vital the increased trans-epithelial resistance was prevented when either cysteine was mutated. PCP was increased after mutation of Cys64. Charoenphandhu et al.34 looked at the effects on expression of Claudin family proteins after (C)hronic (M)etabolic (A)cidosis) in relation to PCP and showed that Claudin-1 through -15, Claudin-17 through -20 and Claduin-22 and Claudin-23 were present in the duodenum of normal rats. Following administration of 1.5%NH94 Cl for 21 d the expression of Claudin-2, Claudin-3, Claudin-6, Claudin-8, Claudin-11, Claudin-12, Claudin-14, Claudin-19 and Claudin-22 were increased significantly, although the expression of Claudin-20 remained unchanged. Thus the results of the present study showing no increase in PCP with upregulation of Claudin-20 would seem to agree with the previous study showing no change in Claudin-20 expression with an increase in PCP. Such results may account for the selective permeability exhibited in barrier function.34
It may be inferred that Claudin-20 has a differential role to play in breast epithelial cells, not just in the function of the TJ complex. A timely review by Webb et al.35 summarized that new functions for Claudins outside TJs was supported by the discovery that the disruption and reduction/loss of the TJ structure occurred during the tumor progression. Moreover, there is a distinct expression of Claudin subtypes.36 Unlike Claudin-5 and Claudin-16 which are significantly reduced in human breast cancer, a number of Claudins are upregulated in cancer.13,14 Claudin-4 is upregulated in breast cancer as are Claudins-3 and -7.37,38
Taken together with our study, showing that Claudin-20 is increased in aggressive breast tumors and being associated with poor patient survival and metastatic disease demonstrates a possible use of Claudin-20 as a target for therapy and as a marker for determining treatment regimens. It is clear that further study is required to explicate the potential of Claudin-20.
Materials and Methods
Reagents and antibodies
Anti-Claudin-20 was obtained from Abnova (Abnova GmbH, H00049861-M01), anti-actin (sc-8432) from Santa-Cruz Biotechnologies Inc. (Santa Cruz, USA), secondary antibody anti-mouse peroxidase conjungated from Sigma (Sigma-Aldrich, A-9044), secondary antibody anti-goat peroxidase conjungated from Sigma (Sigma-Aldrich, A-5420).
Cell lines and culture conditions
The human breast cancer cell lines MDA-MB-231 and MCF7was routinely maintained in Dulbecco’s Modified Eagle Medium/Ham’s F12 (Sigma-Aldrich, D6421) supplemented with 10% fetal calf serum, penicillin and streptomycin. The cells were incubated at 37°C, 5% CO2 and 95% humidity.
Human breast specimens
A total of 133 breast samples were obtained from breast cancer patients (114 breast cancer tissues and 30 associated background or related normal tissue), with the consent of the patients and approved by local ethical committee. The anonymised breast tissue samples were obtained within the guidelines of the appropriate ethics committee (Bro Taf Health Authority 01/4303 and 01/4046). Informed patient consent was not applicable in this instance (as stated in the Human Tissue Act 2004, UK). The pathologist verified normal background and cancer specimens, and it was confirmed that the background samples were free from tumor deposit. These tissues after mastectomy were immediately frozen in liquid nitrogen.
Immunohistochemistry of human breast tissue
Cryostat sections of frozen tissue were cut at 6 µm, placed on Super Frost Plus slides (LSL UK, Rochdale, UK), air-dried and fixed in a 50:50 solution of alcohol:acetone. The sections were then air-dried again and stored at -20°C until used. Immediately before commencement of immuno-staining, the sections were washed in buffer for 5 min and treated with horse serum for 20 min as a blocking agent to non-specific binding. Negative controls were used where necessary. Primary antibodies were used at 1:100 dilution for 60 min and then washed in buffer. The secondary biotinylated antibody at 1:100 dilution (Universal secondary, Vectastain Elite ABC, Vector Laboratories Inc., Burlingham, CA, USA) was added (in horse serum/buffer solution) for 30 min, followed by numerous washings. Avidin/Biotin complex was added for 30 min, again followed with washes. Diaminobenzadine was used as a chromogen to visualize the antibody/antigen complex. Sections were counterstained in Mayer’s hematoxylin for 1 min, dehydrated, cleared and mounted in DPX.
Overexpression of Claudin-20 in MDA-MB-231 in human breast cancer cells
A range of normal human tissues were screened for Claudin-20. Normal placenta tissue was chosen for endogenous expression of Claudin-20. The human breast cancer cell lines MDA-MB-231and MCF7 were chosen for introduction of the Claudin-20 gene. The gene, after amplification from placenta tissue cDNA was cloned into aPEF6/V5-His TOPO TA plasmid vector (Invitrogen Ltd., Paisley, UK) before electroporation into the endothelial cells. Expression of the gene was confirmed by RT-PCR. The Claudin-20 expression construct and empty plasmid were, respectively, used to transfect control cells by electroporation. Stably transfected cells were then used for subsequent assays after being tested at both transcriptional and translational level. Those cells containing the expression plasmid and displaying enhanced Claudin-20 expression were designated MDACL20/MCF7CL20, those containing the closed pEF6 empty plasmid and used as control cells were designated MDApEF6/MCF7pEF6 and unaltered wild type were designated MDAWT/MCF7WT. The expression primers were as follows: Claudin-20 (coding region), Cldn20ExF1 ATGGCCTCAGCAGGACTC and Cldn20ExR1 TTACACATAATCCTTCAGATTG (659 bp).
RNA extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Cells were grown to confluence in a 25cm3 flask before RNA was extracted using total RNA isolation (TRI) reagent and following the protocol provided (Sigma-Aldrich, T9424). RNA was converted to cDNA using iScript cDNA synthesis kit (Primer Design Ltd., Southampton, UK). Following cDNA synthesis, samples were probed using GAPDH primers to check the quality of the cDNA and confirm uniform levels within each sample together with those specific for the Claudin-20 transcript. Conventional PCR was performed using a T-Cy Thermocycler (Breacon Technologies Ltd., The Netherlands) using REDTaq® ReadyMixTM PCR Reaction mix (Sigma-Aldrich, R2523). Cycling conditions were as followed: 94°C for 5 min, 94°C for 30 s, 55°C for 30 s, 72°C for 30 s and the final extension phase at 72°C for 7 min for 36 cycles. The PCR products were separated on a 2% agarose gel and electrophoretically separated. The gel was then stained with ethidium bromide prior to examine under UV light and photographs taken.
Real-time quantitative Polymerase Chain Reaction (Q-PCR)
The assay was based on the Amplifluor system. It was used to detect and quantify transcript copy number of Claudin-20 and CK-19 (to normalize data)39 in tumor and background samples. Primers were designed by Beacon Designer software, which included complementary sequence to universal Z probe (Intergen, Inc.). Each reaction contains 10 pmol reverse primer (which has the Z sequence), 10 pmol of FAM-tagged universal Z probe (Intergen, Inc.) and cDNA (equivalent to 50ng RNA). Sample cDNA was amplified and quantified over a large number of shorter cycles using an iCyclerIQ thermal cycler and detection software (BioRad laboratories, Hammelhempstead, UK) under the following conditions: an initial 5 min 94°C period followed by 60 cycles of 94°C for 10 s, 55°C for 15 s and 72°C for 20 s. Detection of GAPDH copy number within these samples was later used to allow further standardisation and normalization of the samples. The primers for Claudin-20 were as follow: Cldn20F1 AGCAAACTTTCTGGATCTGA and Cldn20ZR ACTGAACCTGACCGTACACAGAAAATCATGCCAGAGAT. Primers for CK-19 were as follows: CK-19F CAGGTCCGAGGTTACTGAC and CK-19ZR ACTGAACCTGACCGTACACACTTTCTGCCAGTGTGTCTTC
Immunofluorescent staining of human breast cancer cells
For immunofluorescence staining, cells were grown in 16-well chamber slides (LAB-TEK International, Sussex, UK) (30,000 cells/well) and incubated in a 37oC/5% incubator for a set period of time (0–24h). After incubation, the culture medium was aspirated, the wells rinsed with balanced salt solution (BSS) buffer and the cells fixed in methanol for 20 min at -20°C. After fixation the cells were washed twice using BSS buffer and permeabilised by the addition of 200 µl of 0.1% Triton X-100 (Sigma-Aldrch Ltd, Poole, UK) detergent in Phosphate buffered solution (PBS) for 5 min at room temperature. Cells were rinsed twice with BSS buffer and 200 µl of blocking buffer (10% horse serum in TBS) was added to each well and the chamber slide incubated for 40 min at room temperature on a bench rocker. The wells were washed once with wash buffer (3% horse serum in TBS buffer containing 0.1% Tween20) and 100 μl of primary antibodies prepared in wash buffer was added to the appropriate wells. The chamber slide was incubated on the rocker for a further 60 min at room temperature. Wells were washed twice with TBS buffer (with 0.1% Tween20) and cells were incubated in 100 µl of secondary antibody (fluorescein isothiocyanate (FITC) conjugate Sigma-Aldrich Ltd, Poole, Dorset, UK) (diluted in the same manner as the primary antibodies) for 50 min. The chamber slide was wrapped in foil to prevent light reaching the conjugate. Finally, the wells were rinsed twice with wash buffer, once in BSS buffer mounted with FluorSavetm (Calbiochem-Novabiochem Ltd, Nottingham, UK) reagent and visualized using an Olympus BX51 microscope with a Hamamatsu (Welwyn Garden City, Herts, UK) Orca ER digital camera at X 100 using oil immersion lens.
SDS-PAGE, western blotting and co-immunoprecipitation
Cells were grow to confluence, detached and lysed in HCMF buffer containing 0.5% SDS, 0.5% Triton X-100, 2Mm CaCl2, 100 µg/ml phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, 1mg/ml aprotinin and 10Mm sodium orthovanadate for 1 h, sample buffer was added and the protein boiled at 100oC for 5 min before being spun at 13,000 g for 10 min to remove insolubles. Protein concentration was quantified using Bio-Rad Protein Assay kit (Bio-Rad Laboratories, 500–0001). Equal amounts of protein from each cell sample were added onto a 10% or 15% (depending on protein size) acrylamide gel and being subjected to electrophoretic separation. The proteins were transferred onto nitrocellulose membranes which were blocked and probed with specific primary antibodies (1:500), following with peroxidase-conjugated secondary antibody (1:1000). Protein bands were visualized with Supersignal West Dura system (ThermoScientific, 34075) and detected using a CCD-UVIprochemin system (UVItec Ltd., Cambridge, UK).
Co-immunoprecipitation samples were prepared as follows: cell lysate of the protein of interest was probed with primary antibody (1:100 dilution) and placed on a rotating wheel for 2 h allowing the Claudin-20 antibody to bind to its target. One hundred microlitres of conjugated A/G protein agarose beads (Santa-Cruz Biotechnologies Inc., USA) were added to each sample to make the antibody-protein complex insoluble, followed by overnight incubation on the rotation wheel. The supernatant was discarded and the pellet was washed in 200µl of lysis buffer and resuspended in 200µl of 2X Lamelli sample buffer concentrate (Sigma-Aldrich, S3041), then denatured for 5 min by boiling at 100°C.
Trans-epithelial resistance (TER)
Cells were seeded into 0.4 μm transparent pore size inserts (Greiner Bio-one, 662641) at a density of 50,000 cells in 200μl of ordinary medium within 24 well plates, grown to confluence, the medium removed and replace with fresh medium containing 15Mm Hepes, L-Glutamine. Medium alone was added to the base of the wells (control) or with 40ng/ml HGF. Resistance across the layer of cells was measured using an EVON volt-ohmmeter (EVON, World Precision Instruments, Aston, Herts, UK), equipped with static electrodes (WPI, FL, USA) for a period of 4 h. Each TER experiment was performed independently at least 3 times.
Paracellular permeability (PCP)
This was determined using fluorescencently labeled dextran FITC-Dextran 40. The cells were prepared and treated as in the TER study, but with the addition of Dextran-40 to the upper chamber. Medium from the lower chamber was collected for intervals up to 4 h and fluorescence from these collections was read on a multichannel fluorescence reader (Denly, Sussex, UK). Each PCP experiment was performed independently at least 3 times.
In vitro cell growth assay
Cells were seeded into a 96 well plate at a density of 3,000 cells/well to obtain density readings after 4 h (day 0), 1 d, 3 d and 4 d. Within each experiment four duplicates were set up and each experiment was performed 3 times. After appropriate incubation periods, cells were fixed in 4% formaldehyde in buffered salt solution for 5–10 min before staining for 10 min with 0.5% (w/v) crystal violet in distilled water. The crystal violet was then extracted from the cells using 10% acetic acid. Absorbance was determined at a wavelength of 540nm on a plate reading spectrophotometer.
In vitro cell matrix adhesion assay
Briefly, 45,000 cells were seeded onto the Matrigel basement (10µg/well) membrane in 200μl of normal medium and incubated at 37°C with 5% CO2 for 40 min. After the incubation period, the medium was aspirated and the membrane washed 5 times with 150μl of BSS to remove the non-attached cells, then fixed in 4% formaldehyde (v/v) in buffered salt solution for 10minutes before being stained in 0.5% crystal violet (w/v) in distilled water. The number of adherent cells were counted from 5 random fields per well and 5 duplicate wells per sample, under a microscope and the assay repeated 3 times.
In vitro invasion cell assay
Cell culture inserts (Greiner Bio-One, 665638) were placed into a 24-well plate using forceps and coated in Matrigel. The working solution of Matrigel was prepared at a concentration of 0.5mg/ml in PCR water, adding 100μl to each insert and allowing to dry overnight. Once dried the inserts were rehydrated in 100μl sterile water for 1 h. The water was then aspirated and cells were seeded in the inserts over the top of the artificial basement membrane at a density of 30.000 cells in 200μl per well. The plates were then incubates for 3 d at 37°C with 5% CO2. After the incubation period, the Matrigel layer together with the non-invasive cells was cleaned from the inside of the insert with a tissue paper. The cells which had migrated through the pores membrane and invaded into the Matrigel, were fixed in 4% formaldehyde (v/v) in BSS for 10 min before being stained in 0.5% crystal violet (w/v) in distilled water. The cells were then visualized under the microscope under X40 magnification, 5 random fields counted and duplicate inserts were set up for each test sample. Each invasion assay was performed in triplicate and in 3 independent experiments.
Electric cell impedence sensing (ECIS)
ECIS (electric cell impedence sensing) instrument (Applied Biophysics Inc., NJ, USA) was used for motility assay (wounding assay), wounding/cell modeling analysis in the study model. The ECIS instrument measures the resistence/impedance and capacitance of cells attached to a gold electrode. The arrays were seeded at a density of 40,000 cells in 400 μl of medium with 15Mm Hepes, L-Glutamine to achieve confluent monolayers following treatment with motility-related inhibitors. After 24 h in culture, the confluence and viability of the cell monolayer was confirmed by a light microscope, thus another electrode check was run to check the impedance value of the array to ensure correct position of the contacts. The monolayer of cells was electrically wounded with a 5V AC at 4,000Hz for 30 s. Impedance and resistance of the cell layer were immediately recorded every millisecond for a period of up to 24 h. Replicates (12) were performed within each experiment, whuch was then repeated 4 times.
In vivo development of mammary tumor
Athymic nude mice (nu/nu) were purchased from Charles River Laboratories (Charles River Laboratories, Kent, UK) and maintained in filter top units according to Home office regulation. Each group of mice consisted of 5 mice and each mouse was injected with a mix of 2x106 cancer cells in 100 µl in a 0.5 mg/ml Matrigel suspension in both flanks. Two groups were included: MDApEF6 control transfected cells, and MDACL20 displaying enhanced Claudin-20 expression. The mice were weighted and the size of the growing tumor measured using vernier callipers under sterile conditions every week. Those mice that developed tumors exceeding 1cm3 or suffered 25% weight loss during the experiment were terminated under Schedule 1 according to the UK Home Office and the UK Coordinating Committee on Cancer Research (UKCCCR) instructions. At the end of the experimental work, animals were weighed, terminated under Schedule 1 and tumors were removed if of sufficient size. Tumour volume was determined, at each point, using the following formula: tumor volume = 0.523 x width2 x length.
Statistical analysis
Results data was analyzed using SigmaPlot software (version 11.0). The statistical comparisons between the test (MDACL20/MCF7CL20) and the control cell lines, using as control wild type cells (MDAWT/MCF7WT) or cells containing a closed pEF6/ V5-His TOPO TA plasmid vector (MDApEF6/MCF7pEF6) were made using a Students two sample t-test. In all cases 95% confidence intervals were used. Kaplan Meier was used to determine patient survival (The patients were divided into those with high levels and those with low levels of Claudin-2 transcripts, with levels from patients who had a moderate prognostic index (NPI-2 group) as the cut-off point.).
Acknowledgments
The authors would like to thank Cancer Research Wales for supporting this work.
Glossary
Abbreviations:
- TJ
Tight Junction
- HGF
hepatocyte growth factor
- NPI
Nottingham prognostic indicator
- TNM
tumour nodal metastasis
- TER
transepithelial resistance
- PCP
paracellular permeability
- ECIS
electric cell impedence sensing
- EMP-1
epithelial membrane protein-1
- TMP
tumour-associated membrane protein
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/26518
References
- 1.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda) 2010;25:16–26. doi: 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 2.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–82. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- 3.Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–91. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Lapointe TK, O’Connor PM, Jones NL, Menard D, Buret AG. Interleukin-1 receptor phosphorylation activates Rho kinase to disrupt human gastric tight junctional claudin-4 during Helicobacter pylori infection. Cell Microbiol. 2010;12:692–703. doi: 10.1111/j.1462-5822.2010.01429.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin TA, Das T, Mansel RE, Jiang WG. Enhanced tight junction function in human breast cancer cells by antioxidant, selenium and polyunsaturated lipid. J Cell Biochem. 2007;101:155–66. doi: 10.1002/jcb.21162. [DOI] [PubMed] [Google Scholar]
- 6.Paschoud S, Bongiovanni M, Pache JC, Citi S. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 2007;20:947–54. doi: 10.1038/modpathol.3800835. [DOI] [PubMed] [Google Scholar]
- 7.Turunen M, Talvensaari-Mattila A, Soini Y, Santala MZ. Claudin-5 overexpression correlates with aggressive behavior in serous ovarian adenocarcinoma. Anticancer Res. 2009;29:5185–9. [PubMed] [Google Scholar]
- 8.Arshad F, Wang L, Sy C, Avraham S, Avraham HK. Blood-brain barrier integrity and breast cancer metastasis to the brain. Patholog Res Int. 2010;2011:920509. doi: 10.4061/2011/920509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci (Landmark Ed) 2011;16:898–936. doi: 10.2741/3726. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo T, Ozawa M. J The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. Cell Sci. 2002;117:1675–85. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 11.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 13.Martin TA, Jiang WG. Claudin-16/paracellin-1, cloning, expression, and its role in tight junction functions in cancer and endothelial cells. Methods Mol Biol. 2011;762:383–407. doi: 10.1007/978-1-61779-185-7_28. [DOI] [PubMed] [Google Scholar]
- 14.Escudero-Esparza A, Jiang WG, Martin TA. Claudin-5 participates in the regulation of endothelial cell motility. Mol Cell Biochem. 2012;362:71–85. doi: 10.1007/s11010-011-1129-2. [DOI] [PubMed] [Google Scholar]
- 15.Arabzadeh A, Troy TC, Turksen K. Changes in the distribution pattern of Claudin tight junction proteins during the progression of mouse skin tumorigenesis. BMC Cancer. 2007;7:196–201. doi: 10.1186/1471-2407-7-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satake S, Semba S, Matsuda Y, Usami Y, Chiba H, Sawada N, Kasuga M, Yokozaki H. Cdx2 transcription factor regulates claudin-3 and claudin-4 expression during intestinal differentiation of gastric carcinoma. Pathol Int. 2008;58:156–63. doi: 10.1111/j.1440-1827.2007.02204.x. [DOI] [PubMed] [Google Scholar]
- 17.Song X, Li X, Tang Y, Chen H, Wong B, Wang J, Chen M. Expression of claudin-2 in the multistage process of gastric carcinogenesis. Histol Histopathol. 2008;23:673–82. doi: 10.14670/HH-23.673. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto K, Kusumi T, Sato F, Kawasaki H, Shibata S, Ohashi M, Hakamada K, Sasaki M, Kijima H. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 2008;29:71–6. doi: 10.2220/biomedres.29.71. [DOI] [PubMed] [Google Scholar]
- 19.Tsunoda S, Smith E, De Young NJ, Wang X, Tian ZQ, Liu JF, Jamieson GG, Drew PA. Methylation of CLDN6, FBN2, RBP1, RBP4, TFPI2, and TMEFF2 in esophageal squamous cell carcinoma. Oncol Rep. 2009;21:1067–73. doi: 10.3892/or_00000325. [DOI] [PubMed] [Google Scholar]
- 20.Lioni M, Brafford P, Andl C, Rustgi A, El-Deiry W, Herlyn M, Smalley KS. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol. 2007;170:709–21. doi: 10.2353/ajpath.2007.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, Futami K, Yamashita Y, Hachimine K, Maekawa S, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A):3729–34. [PubMed] [Google Scholar]
- 22.Huo Q, Kinugasa T, Wang L, Huang J, Zhao J, Shibaguchi H, Kuroki M, Tanaka T, Yamashita Y, Nabeshima K, et al. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 2009;29:851–7. [PubMed] [Google Scholar]
- 23.Gröne J, Weber B, Staub E, Heinze M, Klaman I, Pilarsky C, Hermann K, Castanos-Velez E, Röpcke S, Mann B, et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: frequent dysregulation of claudin-1, -8 and -12. Int J Colorectal Dis. 2007;22:651–9. doi: 10.1007/s00384-006-0197-3. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama F, Semba S, Usami Y, Chiba H, Sawada N, Yokozaki H. Hypermethylation-modulated downregulation of claudin-7 expression promotes the progression of colorectal carcinoma. Pathobiology. 2008;75:177–85. doi: 10.1159/000124978. [DOI] [PubMed] [Google Scholar]
- 25.Oshima T, Kunisaki C, Yoshihara K, Yamada R, Yamamoto N, Sato T, Makino H, Yamagishi S, Nagano Y, Fujii S, et al. Reduced expression of the claudin-7 gene correlates with venous invasion and liver metastasis in colorectal cancer. Oncol Rep. 2008;19:953–9. [PubMed] [Google Scholar]
- 26.Blanchard AA, Skliris GP, Watson PH, Murphy LC, Penner C, Tomes L, Young TL, Leygue E, Myal Y. Claudins 1, 3, and 4 protein expression in ER negative breast cancer correlates with markers of the basal phenotype. Virchows Arch. 2009;454:647–56. doi: 10.1007/s00428-009-0770-6. [DOI] [PubMed] [Google Scholar]
- 27.Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, McBryan J, Jirstrom K, Landberg G, Martin F, Duffy MJ, et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 2009;124:2088–97. doi: 10.1002/ijc.24159. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Medvedev A, Ruzanov P, Marvin KW, Jetten AM. cDNA cloning, genomic structure, and chromosome mapping of the human epithelial membrane protein CL-20 gene (EMP1), a member of the PMP22 family. Genomics. 1997;41:40–8. doi: 10.1006/geno.1997.4524. [DOI] [PubMed] [Google Scholar]
- 29.Rizzolo LJ, Chen X, Weitzman M, Sun R, Zhang H. Analysis of the RPE transcriptome reveals dynamic changes during the development of the outer blood-retinal barrier. Mol Vis. 2007;13:1259–73. [PubMed] [Google Scholar]
- 30.Price MG, Davis CF, Deng F, Burgess DL. The α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor trafficking regulator “stargazin” is related to the claudin family of proteins by Its ability to mediate cell-cell adhesion. J Biol Chem. 2005;280:19711–20. doi: 10.1074/jbc.M500623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain A, Tindell CA, Laux I, Hunter JB, Curran J, Galkin A, Afar DE, Aronson N, Shak S, Natale RB, et al. Epithelial membrane protein-1 is a biomarker of gefitinib resistance. Proc Natl Acad Sci U S A. 2005;102:11858–63. doi: 10.1073/pnas.0502113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piechocki MP, Yoo GH, Dibbley SK, Lonardo F. Breast cancer expressing the activated HER2/neu is sensitive to gefitinib in vitro and in vivo and acquires resistance through a novel point mutation in the HER2/neu. Cancer Res. 2007;67:6825–43. doi: 10.1158/0008-5472.CAN-07-0765. [DOI] [PubMed] [Google Scholar]
- 33.Wen H, Watry DD, Marcondes MC, Fox HS, Fox S. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–17. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoenphandhu N, Wongdee K, Tudpor K, Pandaranandaka J, Krishnamra N. Chronic metabolic acidosis upregulated claudin mRNA expression in the duodenal enterocytes of female rats. Life Sci. 2007;80:1729–37. doi: 10.1016/j.lfs.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 35.Webb PG, Spillman MA, Baumgartner HK. Claudins play a role in normal and tumor cell motility. BMC Cell Biol. 2013;14:19. doi: 10.1186/1471-2121-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kominsky SL, Vali M, Korz D, Gabig TG, Weitzman SA, Argani P, Sukumar S. Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol. 2004;164:1627–33. doi: 10.1016/S0002-9440(10)63721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soini Y. Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma. Hum Pathol. 2004;35:1531–6. doi: 10.1016/j.humpath.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, McBryan J, Jirstrom K, Landberg G, Martin F, Duffy MJ, et al. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 2009;124:2088–97. doi: 10.1002/ijc.24159. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl MW, Hagelei M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol Lett. 2001;23:275–82. doi: 10.1023/A:1005658330108. [DOI] [Google Scholar]