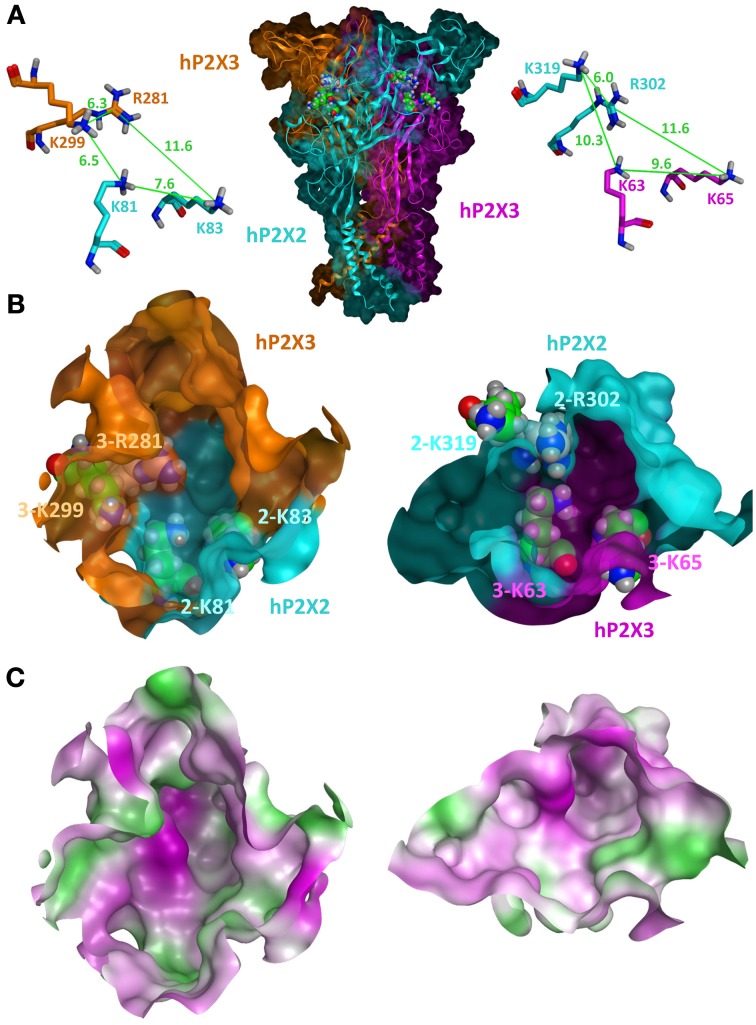
Figure 1.
Homology model of the closed state of the human P2X2/3R. (A) A side view of the heterotrimeric hP2X2(3)2R in which both heteromeric interfaces are visible is shown in the middle. The P2X2 subunit is colored in cyan and the two P2X3 subunits are colored in orange and pink. Selected basic residues important for ATP binding are shown as spheres within the two intersubunit ATP-binding sites. For clarity, the same residues are shown as sticks in a close-up view with depiction of the distances between their side chains. (B) Frontal close-up views of the two heteromeric ATP-binding sites with a partial transparent surface to indicate the orientation of the residues. The coloring of the subunits is the same as in (A). Differences in the three-dimensional structures and volumes of the hydrophilic cavities are clearly seen. (C) Surface representations of the same view as in (B) with gradual depiction of hydrophobic (green) or hydrophilic (pink) areas/residues. Neutral areas are shown gradually white. The hP2X2/3R homology model was generated and visualized by the molecular modeling program MOE2012.10 (Molecular Operating Environment 2012, CCG, Montreal, Canada) using the apo zP2X4 crystal structure (PDB entry 3H9V; (Kawate et al., 2009) as a template as previously described (Wolf et al., 2011; Hausmann et al., 2013).