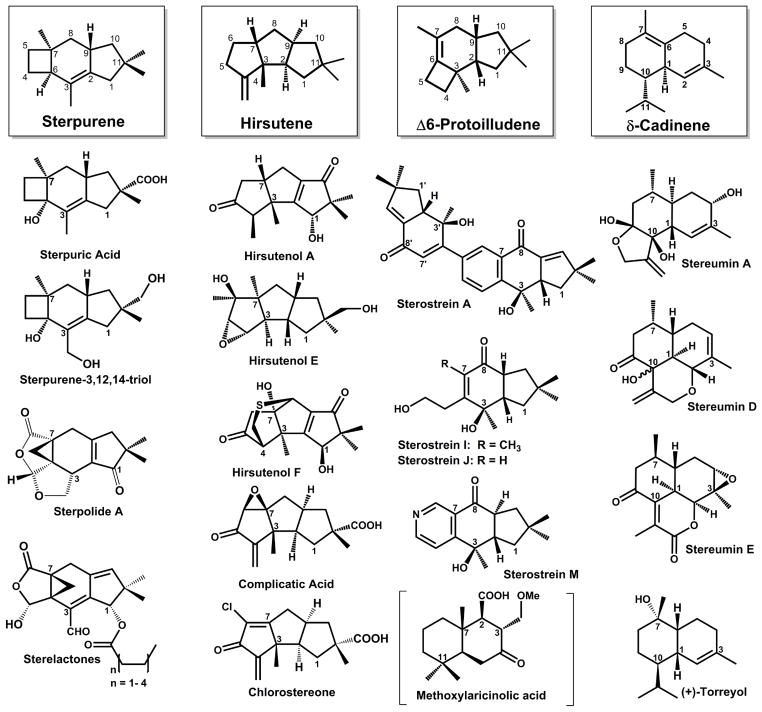
Scheme 1. Representative examples of modified sesquiterpenoids previously isolated from Stereum sp.
Most sesquiterpenoids isolated from Stereum sp. are derived from the trans-humulyl cation, a product of the (2E,6E)-FPP 1,11-cyclization mechanism. Atom numbering is based upon designations in the FPP precursor. Sterpuric acid, sterpolide A, sterpurene-3,12,14-triol, and the sterelactones result from the modification of the sterpurene scaffold.[54–57,67] Modification of the hirsutene scaffold yields hirsutenols A-F,[37,38] as well as complicatic acid and chlorostereone.[36] The sterostreins [32,33] are modified products of the Δ-6 protoilludene scaffold. Additionally, the stereumins are derived from the (3R)-NPP 1,10-cyclization product δ-cadinene,[31,35] (+)-torreyol is a product of attack by water on either the (2E,6E)-FPP 1,10-cyclization intermediate muurolyl cation or the (3R)-NPP 1,10-cyclization intermediate cadinyl-cation.[68] Finally, methoxylaricinolic acid results from a putative 2,7-cyclization product drimane [34,69] (shown in parentheses). Relative stereochemistries of sesquiterpenoids are shown.