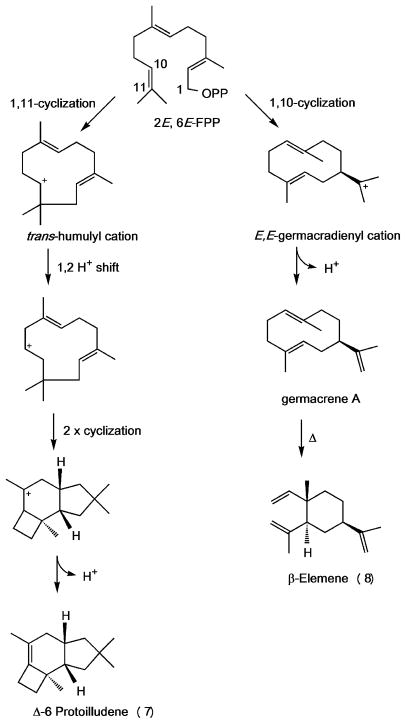
Scheme 2. Cyclization pathways for the production of Δ-6 protoilludene, germacrene A and β-elemene.
A metal ion mediated dephosphorylation of (2E,6E)-FPP yields a reactive carbocation, followed by a specific ring closure. A 1,11-cyclization mechanism produces a trans-humulyl cation, a precursor to a wide range of sesquiterpenes. The trans-humulyl cation can undergo a 1,2 hydride shift followed by two cyclizations and loss of a proton, resulting in Δ-6 protoilludene 7. A 1,10-cyclization mechanism yields a E,E-germacradienyl cation, and loss of a proton leads to germacrene A. β-elemene 8 is the heat induced Cope rearrangement product of germacrene A. Relative stereochemistries of sesquiterpenes are shown.