Abstract
Struma ovarii (SO) is a rare form of ovarian tumor, which is defined by the presence of thyroid tissue comprising more than 50% of the overall tumor volume. The vast majority of the variants of SO are benign; however, malignant tumors have been reported in a small percentage of cases. An aggressive multimodality approach using ovarian cancer staging laparotomy, total thyroidectomy along with radioactive iodine-131 ablation, and thyroxin suppression therapy has been shown to safely treat malignant SO both its initial presentation as well as in the event of any subsequent recurrence with excellent efficacy and possibly better oncological outcomes. The rarity of the disease and the lack of evidence surrounding its management and prognosis continue to remain a challenge to the treating clinician. We present a unique case of malignant SO with an incidental synchronous association of follicular variant of papillary carcinoma of the cervical thyroid gland, this is possibly the second case reported in the English language literature.
Keywords: Iodine-131, papillary carcinoma thyroid, radio iodine therapy, struma ovarii
INTRODUCTION
Struma ovarii (SO) is a rare form of germ cell derived ovarian tumor, which is defined by the presence of thyroid tissue comprising more than 50% of the overall tumor volume. First described in 1899, SO, which literally means ‘goiter of the ovary,’ accounts for just about 1% of all the ovarian neoplasms. The vast majority of the variants of SO are benign (95%); however, malignant tumors have been reported in a small percentage of cases. We present a unique case of malignant SO with an incidental synchronous association of follicular variant of papillary thyroid carcinoma (FVPTC) of the cervical thyroid gland. Only one another similar case has been described in English language literature to the best of our knowledge. Interestingly, the malignant thyroid component of the SO was also FVPTC. We further discuss the controversies surrounding the management of this enigmatic entity.
CASE REPORT
A 51-year-old multigravida with no comorbid illnesses was evaluated at an outside hospital for an abdominopelvic mass, wherein she was administered two cycles of chemotherapy with cisplatin and cyclophosphamide with a presumed clinical diagnosis of an ovarian malignancy. She subsequently defaulted for treatment, only to present to our center, 7 years later with vague complaints of abdominal pain and distension. Clinical evaluation revealed a 12 × 8 cm sized abdominopelvic mass, which could not be made out separately from the uterus on rectal and vaginal examination. General examination and examination of the other systems were normal. A computed tomography (CT) scan of the abdomen and pelvis revealed a 13 × 7.6 cm heterodense adnexal mass with solid and cystic components and punctate calcifications [Figure 1]. The levels of the tumor marker CA-125 was elevated at 85 U/ml. She underwent a staging laparotomy and an optimal cytoreduction (total abdominal hysterectomy, bilateral salpingo-oophorectomy with omentectomy, lymph nodes, and peritoneal sampling) after a frozen section diagnosis of an ovarian teratoma with SO from the right ovary. The final histopathology revealed the mass to be arising from the right ovary [Figure 2] which on microscopy revealed a neoplasm composed of mixed elements arising from all germ layers with predominance of thyroid parenchyma composed of multiple nodules with colloid filled follicles. The nodules within the thyroid tissue measuring 0.5-0.8 mm showed closely packed follicles lined by columnar cells with cell crowding, loss of polarity and nuclear grooves, and features suggestive of FVPTC. The left ovary, uterus, fallopian tubes, omentum, nodes, and the peritoneal biopsies were within normal limits. The final diagnosis suggested an malignant SO [Figure 3]. A total thyroidectomy was subsequently performed, a preoperative ultrasound of the thyroid gland revealed a 4 mm nodule in the right lobe of the thyroid, which was indeterminate on an ultrasound-guided fine needle aspiration cytology (FNAC). The final histopathology of the thyroidectomy specimen revealed the nodule with histological features suggestive of FVPTC. The postoperative iodine-131 whole body scan was negative for metastasis. The patient is on follow-up after remnant ablation for the past 6 months on suppressive doses of eltroxin.
Figure 1.
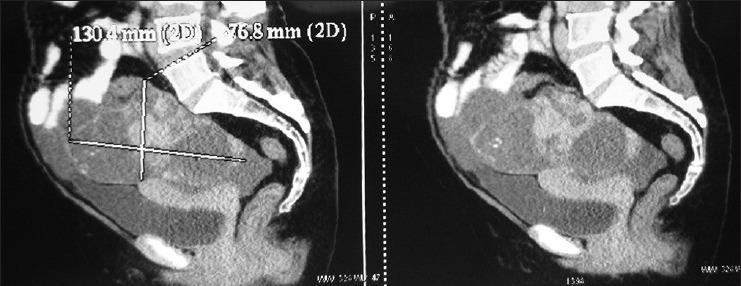
Computed tomography scan of the abdomen and pelvis revealed a 13 × 7.6 cm heterodense adnexal mass with solid and cystic components and punctate calcifications
Figure 2.
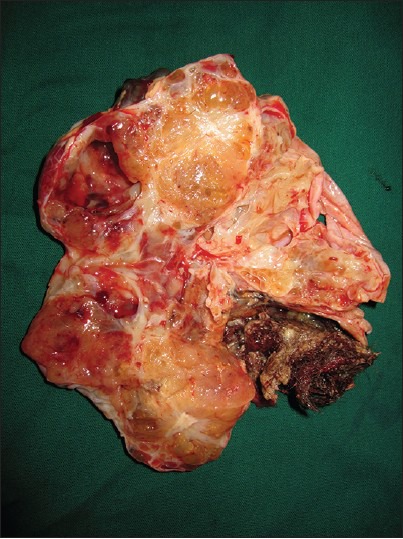
Cut-surface of the right ovary with a solid grey brown mass measuring 10.5 × 8.5 × 6 cm with cystic areas and hair follicles
Figure 3.
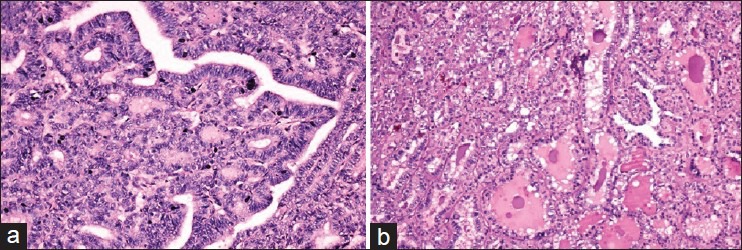
(a) H and E, ×20. Nodules from the right ovary showing closely packed follicles lined by columnar cells with cell crowding, loss of polarity and nuclear grooves, features suggestive of FVPTC. Also seen were hair follicles, adipose tissue, tiny cystic spaces with atrophied lining epithelium, one focus of cyst filled with ciliated columnar epithelium, osteoid, foci of calcification, and large blood vessels. (b) H and E, ×10. Nodule from the right lobe of thyroid showing features sugestive of follicular carcinoma thyroid
DISCUSSION
Although SO has elicited considerable curiosity ever since it was first described, many of its diagnostic and therapeutic aspects continue to remain unclear. Most cases are incidentally discovered, the clinical features of SO are hence obtained mainly from retrospective case reports/series.[1]
The symptoms of ‘SO’ are no different when compared to the other ovarian tumors, being largely nonspecific. Despite containing thyroid tissue, only about 5-8% of SO have features of hyperthyroidism, with ascites being reported in about one-third of the cases[1] Although SO has been described to have characteristic radiological findings; the final diagnosis is invariably made upon histological examination of the resected ovarian tumor.[2] Pathological examination reveals ‘thyroid tissue’ as the major component of the mass which is morphologically and biochemically similar to that of cervical thyroid gland. The pathological criteria used for diagnosing carcinoma of the thyroid gland are widely accepted as the standard for diagnosing malignant SO as well.[3] The malignant thyroid tissue may be of the papillary, follicular variant of papillary carcinoma, or the follicular pattern.
Surgical resection of the ovary is sufficient to treat benign, unilateral SO. A paucity of evidence exists in the literature regarding the management in cases with evidence of malignancy. A total abdominal hysterectomy plus bilateral salpingo-oophorectomy with omentectomy and lymph node sampling seems a reasonable option for woman with malignant SO who have completed their family, although a few reports of fertility sparing surgery with a strict follow-up have also been documented.
The opinion is clearly divided regarding the further course of treatment, between conservative management (observation) alone versus aggressive multimodality management with an added prophylactic total thyroidectomy and adjuvant radioiodine ablation. The conservative group believes that total thyroidectomy followed by iodine-131 radioablation therapy should be reserved only for patients with recurrence or for residual disease.
A multimodal approach to the treatment of malignant SO is favored by many authors in an attempt to decrease the risk of recurrence and to improve the overall survival.[2,4,5,6,7,8] A prophylactic thyroidectomy is imperative before adjuvant treatment to potentiate the effects of radioablation. This approach additionally facilitates the detection of tumor recurrence/metastases during follow-up, radioiodine-131 possesses therapeutic implications as well.[7,8] Thyroglobulin is a well-established marker for monitoring the recurrence of malignancy for thyroid carcinoma; it can be used for monitoring recurrences in patients with malignant SO as well.
An added benefit of a thyroidectomy would be that it would provide pathological confirmation that the struma is indeed ovarian in origin. Metastatic thyroid carcinoma of the ovary is believed to be extraordinarily uncommon and has been reported to occur many years after the primary thyroid carcinoma and may also histologically resemble primary thyroid-type carcinoma arising in SO. The detection of a synchronous thyroid gland primary was a surprising finding in our patient, to the best of our knowledge, only one similar case has been reported prior in the English language literature.[9] The presence of all three germ cell layers in the SO of our patient rules out the possibility of a primary tumor in the thyroid with synchronous ovarian metastasis.
The biological behavior of malignant SO is unpredictable, although the clinical course is believed to be protracted, with a majority of the patients having long-term survival.[3] In an analysis of 88 patients with malignant SO, several factors which included a papillary histology, a struma component of 12 cm or more, adhesions, peritoneal fluid of a liter or more, an ovarian serosal rent, or were identified as being associated with recurrence.[10]
CONCLUSION
An aggressive multimodality approach using ovarian cancer staging laparotomy, total thyroidectomy along with radioactive iodine-131 ablation and thyroxin suppression therapy has been shown to safely treat malignant SO, both its initial presentation as well as in the event of any subsequent recurrence with excellent efficacy and possibly better oncological outcomes. The rarity of the disease and the lack of evidence surrounding its management and prognosis continue to remain a challenge to the treating clinician.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS. Clinical characteristics of struma ovarii. J Gynecol Oncol. 2008;19:135–8. doi: 10.3802/jgo.2008.19.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung SI, Kim YJ, Lee MW, Jeon HJ, Choi JS, Moon MH. Struma ovarii: CT findings. Abdom Imaging. 2008;33:740–3. doi: 10.1007/s00261-008-9362-1. [DOI] [PubMed] [Google Scholar]
- 3.Shaco-Levy R, Peng RY, Snyder MJ, Osmond GW, Veras E, Bean SM, et al. Malignant struma ovarii: A blinded study of 86 cases assessing which histologic features correlate with aggressive clinical behavior. Arch Pathol Lab Med. 2012;136:172–8. doi: 10.5858/arpa.2011-0092-OA. [DOI] [PubMed] [Google Scholar]
- 4.Shrimali RK, Shaikh G, Reed NS. Malignant struma ovarii: The west of Scotland experience and review of literature with focus on postoperative management. J Med Imaging Radiat Oncol. 2012;56:478–82. doi: 10.1111/j.1754-9485.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 5.Jean S, Tanyi JL, Montone K, McGrath C, Lage-Alvarez MM, Chu CS. Papillary thyroid cancer arising in struma ovarii. J Obstet Gynaecol. 2012;32:222–6. doi: 10.3109/01443615.2011.645921. [DOI] [PubMed] [Google Scholar]
- 6.Matysiak-Grzes M, Fischbach J, Gut P, Klimowicz A, Gryczynska M, Wasko R, et al. Struma ovarii maligna. Neuro Endocrinol Lett. 2013;34:97–101. [PubMed] [Google Scholar]
- 7.Steinman RA, De Castro IO, Shrayyef M, Chengazi V, Giampoli E, Van Der Sloot P, et al. Two cases of malignant struma ovarii with metastasis to pelvic bone. Gynecol Obstet Invest. 2013;75:139–44. doi: 10.1159/000345863. [DOI] [PubMed] [Google Scholar]
- 8.DeSimone CP, Lele SM, Modesitt SC. Malignant struma ovarii: A case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol. 2003;89:543–8. doi: 10.1016/s0090-8258(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 9.Marti JL, Clark VE, Harper H, Chhieng DC, Sosa JA, Roman SA. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: A series of 4 patients and a review of 53 reported cases. Thyroid. 2012;22:400–6. doi: 10.1089/thy.2011.0162. [DOI] [PubMed] [Google Scholar]
- 10.Robboy SJ, Shaco-Levy R, Peng RY, Snyder MJ, Donahue J, Bentley RC, et al. Malignant struma ovarii: An analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol. 2009;28:405–22. doi: 10.1097/PGP.0b013e3181a27777. [DOI] [PubMed] [Google Scholar]


