Figure 6.
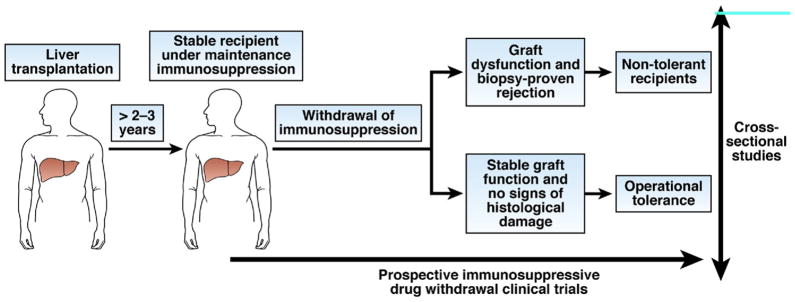
Identification of biomarkers of operational tolerance in clinical transplantation. The search for diagnostic biomarkers of operational tolerance has been conducted mainly in cross-sectional case-control studies, in which peripheral blood samples collected from recipients who became tolerant to transplanted organs after ending immunosuppressive therapy were compared with samples collected from control recipients. In liver transplantation, stable recipients under maintenance immunosuppression with the history of a previous failed attempt at drug withdrawal are used as the control group; in these recipients, the absence of tolerance has been established. Patients who failed to induce tolerance after kidney transplantation and drug withdrawal are not available, so stable recipients who receive maintenance immunosuppression or patients with chronic humoral rejection are used as the control group. In these studies, the confounding effects of pharmacologic immunosuppression are of concern; biomarker discovery trials should be designed as prospective studies in which immune monitoring analyses are performed before immunosuppressive drugs are discontinued. These types of studies have only been attempted with patients undergoing liver transplantation.
