Abstract
The objectives of this research were to evaluate antioxidant activities and nutritional components, including phenolic acid, catechin, organic acid, sugar, and amino acid, of persimmon juice from persimmons grown in different regions around Korea. Persimmon (Diospyros kaki) exhibits potent antioxidant effects in DPPH, ABTS, reducing power, and FRAP methods of analysis. The levels of nutritional constituents showed significant differences among all the samples. In particular, tartaric acid, glucose, gallic acid, epicatechin gallate and aspartic acid were observed to be the predominant component for each of their general chemical groups, with total average contents of 1876.51 mg/kg, 62.69 g/kg, 12.73 mg/kg, 208.99 mg/kg, and 31.84 mg/100 g, respectively. Interestingly, persimmons from the Hadong region presented the highest sugar (130.60 g/kg), phenolic acid (42.27 mg/kg), and catechin (527.97 mg/kg) contents in comparison with other regional samples. Moreover, this location exhibited the greatest antioxidant activity with highest total phenolic (298.01 mg GAE/kg) and flavonoid (32.11 mg/kg RE) contents. Our results suggest that strong antioxidant activities of persimmons correlate with high phenolic acid and catechin contents, particularly gallic acid and epicatechin gallate. Additionally, these two compounds may be key factors when considering the useful ingredients of persimmon.
Keywords: persimmon, fruit juice, antioxidant activity, nutritional constituent, phenolic acid, catechin
INTRODUCTION
The nutritional properties and bioactive compositions (such as amino acids, sugars, phenolic compounds, and organic acids) of natural dietary plants, crops, fruits, and vegetables play an important role in human nutrition and health as potential sources of functional foods and nutraceuticals (1–4). These various nutritional constituents, including organic acids, sugars, and amino acids, are good sources of primary metabolites and are well known as chemical markers for ripeness, taste, flavor, and quality (5). Organic acids have a protective role against chronic diseases that result from antioxidant activities (6). Amino acids provide several health benefits, such as antimutagenicity, antiobesity, and antitumor effects, as well as the reduction of both blood cholesterol (7) and sugars, and they are also essential agents in sweetening and food preservation (8). Phenolic compounds of secondary metabolites are widely distributed in natural edible plants and food products, and they display possession of biological activities, including some health-promoting properties (1,9,10). These compounds are of great interest in the food industry because of what they can add to the nutritional and overall quality value of foods (11). Nowadays, there is considerable evidence that antioxidants in edible sources play a crucial role in the maintenance of health and in prevention of diseases. Especially, natural antioxidants have attracted an increasing interest due to the safety concerns regarding the synthetic antioxidants BHA and BHT. For this reason, many researchers have focused on natural materials that could provide bioactive compounds to prevent numerous diseases.
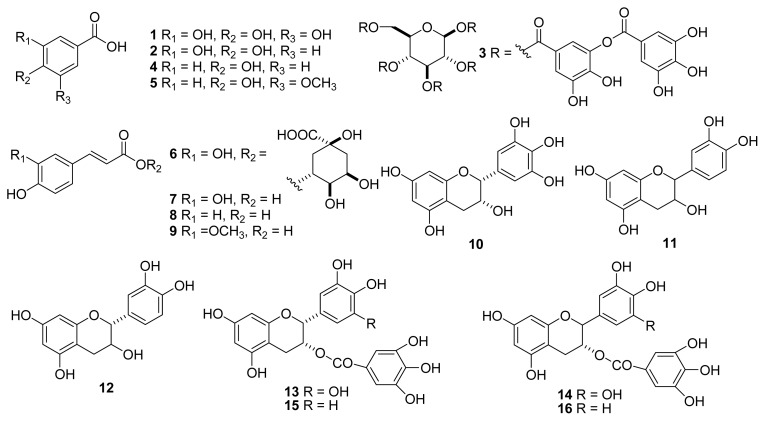
Persimmon (Diospyros kaki L.) belongs to the family of Ebenaceae, and is a familiar fruit throughout Asian countries that has also been widely used for medicinal purposes (12,13). The leaves and fruits of this species have been used throughout history to cure cough, arteriosclerosis, hypertension, and apoplexy (14–16). More recently, the flesh, peel, and leaves of persimmon have received unprecedented attention due to the various biological activities such as antioxidant, antitumor, anti-hyperlipidemia, and antidiabete effects (3,15). Persimmon fruits are associated with various biological activities, including antioxidant effects, and their potent radical scavengers were found to result from the flavonoid groups, namely catechin derivatives (3) (Fig. 1).
Fig. 1.
Chemical structures of phenolic acids (1~9) and catechin (10~16). 1: gallic acid, 2: protocatechuic acid, 3: tannic acid, 4: p-hydroxylbenzoic acid, 5: vanillic acid, 6: chlorogenic acid, 7: caffeic acid, 8: p-coumaric acid, 9: ferulic acid, 10: epigallocatechin, 11: catechin, 12: epicatechin, 13: epigallocatechin gallate, 14: gallocatechin gallate, 15: epicatechin gallate, 16: catechin gallate.
In recent years, there is a growing interest in fruit juices because of the possible nutraceuticals contained therein (17), and many studies have been carried out on a larger scale using clarified fruit juice from a wide variety of sources. Among these many fruit sources, persimmon, in particular, showed beneficial health effects due to several nutritional constituents (12–16,18). Additionally, this species showed potent scavenging action against active oxygen free radicals due to the flavan-3-ols (3). Although numerous researchers revealed that the biological activities of persimmon are due to phytochemicals (3), there is little information available regarding the changes in various components of this species that occur in different geographical regions. Moreover, there is no report of comparative studies on various nutritional constituents and antioxidant activities.
Consequently, we report here for the first time a comparative analysis of five nutritional constituent contents (organic acid, amino acid, sugar, phenolic acid, and catechin) in the fruit juice of persimmon from different regions. We also evaluated for the first time the persimmon juice antioxidant capacities using four different methods (DPPH, ABTS, reducing power, and FRAP), as well as total phenolic and flavonoid contents.
MATERIALS AND METHODS
Plant material and chemicals
Persimmon fruits (D. kaki, cv Gapjubaekmok) used in the study were grown in six different regions around Korea: Sancheong, Hadong, Sacheon, Jinju, Yeongam, and Gurye. The samples of each region were collected from 5 sites. The fruits of persimmon were harvested in 2009. These above regions are well known for having the greatest persimmon harvest in Korea. The acetic acid, water, methanol, and acetonitrile used in the HPLC analysis were purchased from Merck (Darmstadt, Germany). Nine standard phenolic acids including gallic, protocatechuic, tannic, p-hydroxybenzoic, vanillic, chlorogenic, caffeic, p-coumaric, and ferulic acids were purchased from Sigma Chemical Co. (St. Louis, MO, USA) (Fig. 1). Seven standard catechins, including catechin, catechin gallate, epicatechin, epicatechin gallate, gallocatechin gallate, epigallocatechin, and epigallocatechin gallate were also obtained from Sigma Chemical Co. (Fig. 1). The compositions of organic acid, sugar, and amino acid were purchased from Merck. Folin-Ciocalteu’s phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), butylated hydroxytoluene (BHT), potassium ferricyanide, potassium persulphate, trichloroacetic acid (TCA) were also purchased from Sigma Chemical Co.
Preparation of persimmon fruit juice and its methanol extract
The fruit juice of persimmon was prepared through a simple process of solid and liquid separation (19). After the core of the persimmon fruit was removed, the remaining materials were centrifuged at 5,000 rpm for 10 min. The supernatant of persimmon fruit juice was gathered in round flask. Furthermore, this supernatant was freeze-dried at −70°C and then the residue was extracted with methanol.
Preparation of the extracts for antioxidant activities and nutritional constituents
The freeze-dried residue of persimmon fruit juice was extracted with methanol for two days at room temperature. Antioxidant activity, phenolic acid, catechin, amino acid, total phenolic content, and total flavonoid content were measured from the methanol extract. Organic acid and sugar analyses were carried out using the fresh persimmon fruit juice.
DPPH radical scavenging activity
The DPPH radical scavenging activities in the methanol extracts of all samples were measured by the method described by Lee et al. (9) with a slight modification. Briefly, sample extracts (0.1 mL) at various concentrations were added to both 0.49 mL of methanol and 0.39 mL of a DPPH methanolic solution (1 mM). The mixtures were vigorously vortexed and incubated for 30 min at room temperature in the dark. The absorbance of mixtures was determined by using a Beckman DU650 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). BHT was used as the reference. The scavenging activity was expressed as a percentage using the following formula:
ABTS•+ radical scavenging activity
This assay was based on the ability of different substances to scavenge the ABTS•+ radical cation in comparison to a standard (BHT). The ABTS•+ was dissolved in ethanol for a final concentration of 7 mM. This radical cation was produced by reacting the ABTS stock solution with 2.45 mM potassium persulphate and leaving the mixture for 10 to 14 hr until the reaction was complete and absorbance was stable. The ABTS•+ stock solution was diluted in ethanol to an absorbance of 0.70 at 734 nm for measurement (Beckman Coulter DU650). After the addition of 0.9 mL of diluted ABTS•+ to 0.1 mL of the methanol extract, the absorbance was taken 3 min after the initial mixing (9). This scavenging activity (%) was expressed as a percentage using the following formula:
Reducing power
The reducing power was determined according to the method of Siddhuraju & Becker (20). The methanol extract of sample (2 mL) was mixed with 1 mL of phosphate buffer (0.2 M and pH 6.6) and 2 mL of 1% potassium ferricyanide. After the mixture was incubated at 50°C for 20 min, 2 mL of 10% TCA, 2 mL of distilled water, and 0.4 mL 1% ferric chloride were added and the absorbance was then measured at 700 nm (Beckman Coulter DU650). Reducing power of BHT was also measured for a comparison.
Ferric reducing antioxidant power (FRAP)
The FRAP assay was done according to Benzie and Strain (21) with some modifications. The FRAP reagent included 300 mM acetate buffer, 10 mM 2,4,6-tripyr-idyl-s-triazine (pH 3.6) solution in 40 mM HCl, and 20 mM FeCl3 solution in a 10:1:1 ratio. The 300 mM acetate buffer was prepared by mixing 3.1 g of sodium acetate trihydrate with 16 mL glacial acetic acid and brought to 1 L with distilled water. This assay was performed by warming 1 mL of distilled water to 37°C before adding 25 μL sample and 1 mL of reagent and incubating at 37°C for 4 min reaction. The absorbance at 593 nm was measured relative to a reagent blank. The total anti-oxidant activity in the methanol extract of sample was determined against a standard of known FRAP value.
Determination of total phenolic content
The total phenolic content of the methanol extract was determined using the Folin-Ciocalteau method (10). Briefly, each sample (500 μL was added to 250 μL 2 N Folin-Ciocalteau reagent. After 5 min, 500 μL of 7% Na2CO3 solution was added with mixing. After 1 hr at room temperature, the absorbance at 750 nm (Beckman Coulter DU650) was measured. The standard curve for total phenolics was made using gallic acid solutions (0, 50, 100, 250, and 500 mg/L) with the same procedure as above. The results were expressed as mg gallic acid equivalents (GAE/g) extract.
Determination of total flavonoid content
The total flavonoid content was determined using a modified colorimetric method (1). The diluted extract (1 mL) as added to a test tube containing 7 mL of methanol. After addition of 2 mL of 90% diethylene glycol, the reaction mixture was initiated by adding 0.1 mL of 4 M sodium hydroxide. After heating for 30 min at 50°C, the reaction mixture was incubated at room temperature for 30 min. The absorbance of the solution was measured at 420 nm (Beckman Coulter DU650). Total flavonoid content was determined using a standard curve of rutin (0, 10, 25, and 50 mg/L) and then expressed as mg rutin equivalents (RE/g) extract.
Organic acid and sugar analyses
For the contents of sugar and organic acid from the fruit juice of persimmon, 10 g sample was extracted with 60 mL of twice-distilled water and kept for 2 hr at room temperature with stirring. The extracted sample was centrifuged at 5000 rpm for 5 min at room temperature. The supernatant was filtered through a 0.45 μm syringe filter (Whatman Inc., Maidstone, UK). The contents of organic acids were determined by HPLC analysis using an Agilent 1200 series (Agilent Co., Forest Hill, Vic, Australia). A sample of the crude organic acid extract was injected onto a TSKgel ODS-100W column (4.6 mm × 250 mm, 5 μm, Tosoh Corp., Tokyo, Japan) and the column temperature was set to 40°C. For the mobile phase, 0.1% phosphoric acid was used and its flow rate was 1.0 mL/min. The volume of injection was 20 μL and organic acid peaks were monitored at 210 nm. The determination of sugars was carried out using HPLC (Dionex Co., Sunnyvale, TX, USA) with a refractive index (RI) detector (Shodex RI-71). The separation was performed by using a Waters carbohydrate analysis column (3.9 × 300 mm, 10 μm). The column temperature was kept at 65°C and the injection volume of sample was 20 μL, with a flow rate of 0.7 mL/min.
Phenolic acid and catechin analyses
The fruit juice (1 g) was extracted with methanol (20 mL) and mixed with a vortex at room temperature for 5 hr. The extract was centrifuged at 5,000 rpm for 5 min at room temperature, and the supernatant was then filtered through a 0.45 μm syringe filter (Whatman Inc.). The filtrate (20 μL) of solvent extract was injected onto an analytical reverse phase C18 column (4.6 mm × 250 mm, XTerra™ RP, 5 μm, Waters Corp., Milford, MA, USA) by HPLC (Agilent 1200 series, Agilent Co.). The mobile phase was composed of 0.5% acetic acid in water (A) and methanol (B). The gradient program was as follows: 0% B to 10% B (5 min), 10% B to 20% B (10 min), 20% B to 30% B (10 min), 30% B to 40% B (20 min), 40% B to 60% B (10 min), 60% B to 80% B (5 min), 80% B to 100% B (5 min), and then maintained for 10 min before returning to the initial conditions. Total retention time was 60 min. The detection was made at 280 nm.
Amino acid analysis
To analyze amino acid in persimmon juice, the concentrated methanol extract (1.0 g) was suspended in 10 mL of 0.1 M HCl in an ultrasonic bath for 20 min and then passed through a 0.45 μm syringe filter. Derivatisation was carried out by using dabsyl chloride for 65°C for 30 min. The reaction mixture was left for 10 min at room temperature and diluted with deionized water before HPLC analysis. HPLC was performed using an Agilent 1200 series (Agilent Co.). The solvent flow rate was 0.5 mL/min using a gradient made with elution A, consisting of 0.14 M sodium acetate 3H2O, 0.1% triethylamine, titrated to pH 6.4 with phosphoric acid and elution B, 60% acetonitrile. The gradient program was as follows: 0~5 min, 16% B; 13 min, 26% B; 25 min, 29% B; 55 min, 75% B and then held for 10 min before returning to the initial conditions. The column temperature was 50°C and the volume of injection was 2 μL. The detector was set at 430 nm.
Statistical analysis
All the measurements were made in triplicate. The results were subject to variance analysis using Sigma plot 2001 (Systat Software Inc., Chicago, IL, USA) and nutritional constituent contents were expressed as mean ± SD (standard deviations).
RESULTS AND DISCUSSION
Antioxidant activity
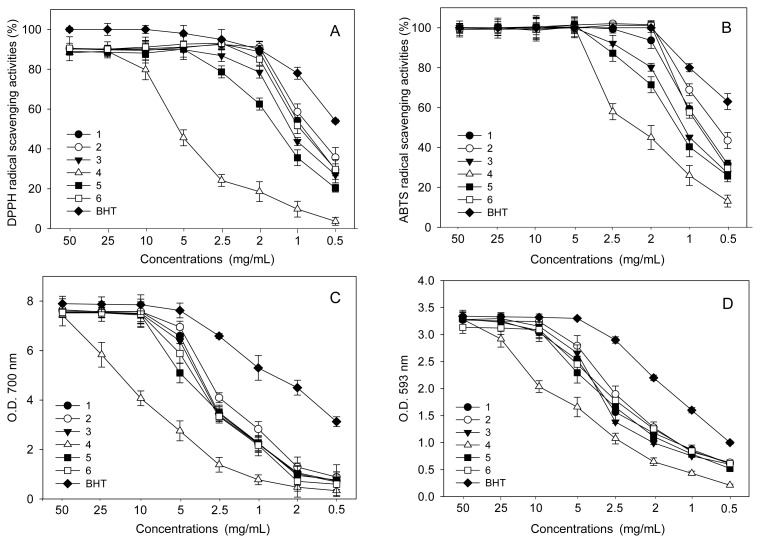
Beneficial effects on health in foods and traditional medicines may be ascribed to the presence of natural antioxidants, which can scavenge oxygen radicals and inhibit peroxidation (22). The crude extracts of numerous natural plants are of increasing interest in the food and medicine industries, because they can be used in the treatment of various diseases and improve the nutritive values of foods. To measure antioxidant activities in the methanol extract of persimmon fruit juice, we employed various methods, including DPPH radical scavenging activity, ABTS radical scavenging activity, reducing power, and FRAP. In the present study, sample concentrations were evaluated by preliminary experiments to determine concentrations needed to demonstrate the effects. As shown in Fig. 1, antioxidant activities were investigated using eight different concentrations (0.5, 1, 2, 2.5, 5, 10, 25, and 50 mg/mL).
DPPH radical scavenging activity
The DPPH radical scavenging method is commonly used to measure the total antioxidant status because of its reproducibility and simple quality control (9). Moreover, this activity has been used extensively in the screening of antioxidants from vegetables, crops, fruits, and natural plants (1,22). Thus, this effect in persimmon juice was estimated by the percentage inhibition of radical formation from the methanol extract of each sample and BHT (positive control). Fig. 2A depicts the DPPH radical scavenging activities in a dose-dependent manner at 0.5~50 mg/mL. The effects of BHT and the sample extracts increased with increasing concentrations. Although the activities of all extracts were lower than those of BHT, the extracts in different regions possessed variability in scavenging activities against this radical. As shown in Fig. 2A, the DPPH radical scavenging activities in the methanol extracts were similar to those of BHT at concentrations of 25 and 50 mg/mL while the remaining concentrations showed slight differences. Notably, this activity showed predominant differences at 0.5 ~2.5 mg/mL. Furthermore, the methanol extract of the Jinju sample exhibited the lowest antioxidant activity, whereas the highest activity was seen in the samples from the Hadong region (Fig. 2A). These observations suggest that the fruit juice of persimmon in Hadong has the highest phenolic content, because linear correlations between phenolic compound content and DPPH radical scavenging activity has been previously demonstrated (22). Based on the above-mentioned considerations, we are convinced that fruit juice of Hadong sample are potent free radical scavengers and may be used as a good source of natural antioxidants for food and commercial uses in comparison with other samples.
Fig. 2.
Comparison of antioxidant activities concerning various concentrations in the methanol extracts from persimmon juice harvested from different regions of Korea. 1, Sancheong; 2, Hadong; 3, Sacheon; 4, Jinju; 5, Yeongam; 6, Gurye. (A) DPPH radical scavenging activity; (B) ABTS radical scavenging activity; (C) Reducing power; (D) FRAP.
ABTS•+ radical scavenging activity
Another widely used method to determine free-radical scavenging activity is based on the scavenging of ABTS radical cation (9). The scavenging activities of the extracts on the ABTS free radical generated by potassium persulphate were compared to standard BHT. The ABTS•+ radical scavenging activities of six different samples were also estimated by comparing the percentage inhibition of ABTS•+ radicals. As can be seen in Fig. 2B, the activities of all samples were similar to those of BHT between 5 and 50 mg/mL, however, the remaining concentrations (0.5~2.5 mg/mL) exhibited ABTS radical scavenging activities of differing levels in a dose dependent manner. Notably, the extracts of all samples showed a scavenging activity level of at least 55% at a concentration of 2.5 mg/mL. Among the six different samples, Hadong showed the highest ABTS•+ radical scavenging activity, while Jinju exhibited the lowest (Fig. 2B). The ability of the persimmon juice extracts to scavenge ABTS radicals were in the order of Hadong> Sancheong> Gurye> Sacheon> Yeongam> Jinju at concentrations of 0.5~50 mg/mL. Based on our experiments, ABTS radical scavenging activities showed significant differences in the same cultivar under different region samples. The results of this assay were similar to those of DPPH radical scavenging activities. However, ABTS radical scavenging activities were higher than those of DPPH at the same concentrations. Generally, it is well-known that the extracts of natural plants have a higher scavenging activities with the ABTS•+ radical when compared to the DPPH radical (9). Our results suggest that antioxidant compounds in the methanol extract of persimmon fruit juice might be slightly higher ABTS•+ radical scavengers than those of DPPH radical. Also, the differences of anti-oxidant activities may be due to the phenolic compound contents, as described in an earlier report (22).
Reducing power
The reducing power assay compares antioxidants based on their ability to reduce ferric (Fe3+) to ferrous (Fe2+) ion through the donation of an electron, with the resulting ferrous ion (Fe2+) production measured by the formation of Pearl’s Prussian blue at 700 nm. The reducing powers of the methanol extracts in the fruit juice of persimmon and positive control (BHT) using the potassium ferricyanide reduction method are depicted in Fig. 2C. Although the activities of all extracts were lower than those of BHT, the reducing powers also increased with the increase of their concentrations, similar to what was seen with DPPH and ABTS radicals. The Hadong sample had the strongest reducing power, exhibiting approximately twice as much activity between 1 and 10 mg/mL as the Jinju sample, which displayed the lowest reducing power. Moreover, at a concentration of 5 mg/mL, all regional samples exhibited good reducing power, with the following values for each: 7.12 (Hadong) 6.94 (Sancheong), 6.81 (Sacheon), 5.92 (Gurye), 5.27 (Yeongam), and 2.95 (Jinju) (Fig. 2C). Previous researchers have reported that the reducing power of bioactive compounds was associated with the antioxidant activity (20). For this reason, the Hadong sample may be the highest in phenolic compound contents by comparison those of other region samples. Furthermore, it is well established that this assay was associated with the presence of reductones (23). Therefore, it is presumed that persimmon fruits have abilities to react with free radicals to convert them into more stable non-reactive species and to determine radical chain reaction.
FRAP activity
The FRAP assay determines the reducing potential of an antioxidant reacting with a ferric tripyridyltriazine (Fe3+-TPTZ) complex and producing a colored ferric tripyridyltriazine (Fe2+-TPTZ) (21). The intensity of the color is related to the amount of antioxidant reductants in the extract of sample. This assay is also commonly used for the routine analysis of food and natural plant extracts (24). The trend for ferric ion-reducing capacities of the methanol extracts in fruit juice of the harvested persimmon are shown in Fig. 2D. The activities of all extracts were somewhat less effective than BHT. The highest FRAP effect was detected in Hadong sample, whereas Jinju showed the lowest activity. This result was similar to those of radical scavenging activities and reducing power. From this point, it was confirmed that Hadong sample possessed the highest content of anti-oxidant compounds, in agreement with previously reported data (25). In this study, the differences in FRAP abilities at a concentration of 5 mg/mL were as follows: Hadong (2.86), Sacheon (2.70), Sancheong (2.62), Gurye (2.50), Yeongam (2.37), and Jinju (1.70). Therefore, we suggest that phenolic compounds in persimmon fruit juice may be capable of reducing ferric ions.
Comparison of total phenolic and flavonoid contents
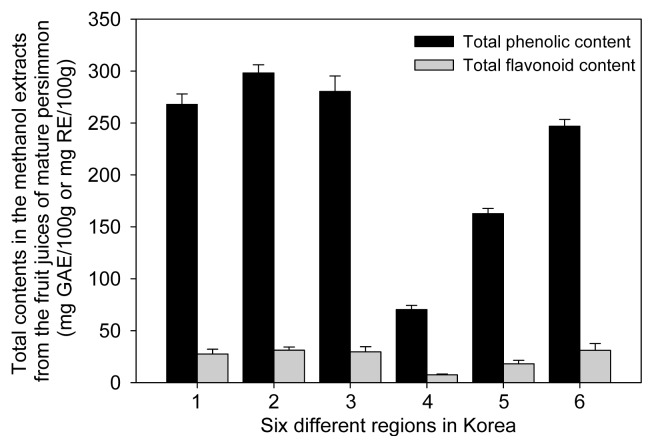
The total phenolic content was measured through gallic acid equivalents (GAE) as described by Folin-Ciocalteau’s colorimetric method with modifications (10). As shown in Fig. 3, total phenolic contents in the fruit juice of persimmon analyzed in the study ranged from 70.44 to 298.01 mg GAE/100 g, using the standard curve of gallic acid (r2=0.998). As with the other assays, differences were observed in the samples from each region. The highest phenolic content was obtained from Hadong with 298.01 mg GAE/100 g, which was approximately 4 times higher than Jinju, which had the lowest content (70.44 mg GAE/100 g). For the other samples, total phenolic contents were in the following order: Sancheong (267.40 mg GAE/100 g) > Sacheon (283.71 mg GAE/100 g) > Gurye (247.54 mg GAE/100 g) > Yeongam (160.83 mg GAE/100 g). Our results appear similar to the patterns shown in a previous study (13). Total phenolic contents of all samples were correlated with antioxidant methods such as radical scavenging effect (DPPH and ABTS), reducing power, and FRAP. Phenolic compounds are also attributed to antioxidant activities of foods, crops, vegetables, and natural plants (1,22,26). Therefore, our results suggest that total phenolic content may contribute significantly to the antioxidant capacity in the fruit juice of persimmon. The differences of total phenolic content in same cultivar may result from the fact that phenolic compounds may be affected by a variety of factors, including light, temperature, humidity, drought, and agronomic conditions in the different harvest regions, as shown in the results of previous literature (20). Generally, flavonoids, including flavanones, flavones, flavanols, and anthocyanins are the most important aromatic secondary metabolites and their consumption has been linked to protection against cancer, heart disease, and oxidants (27). Because of these protective effects, the food industry is increasingly interested in edible sources of high flavonoid contents. In the present study, using the standard curve of rutin (r2 =0.999), total flavonoid contents in persimmon fruit juice from different regions varied from 7.57 to 31.22 mg RE/100 g as shown in Fig. 3. Total flavonoid contents exhibited slight differences in comparison with those of total phenolic content. Among the different samples, the highest flavonoid content was exhibited by the extract of Hadong with 31.22 mg RE/100 g, whereas the lowest content was Jinju sample (7.57 mg RE/100 g). The contents of the remaining samples were in the following order: Gurye (31.09 mg RE/100 g) > Sacheon (29.59 mg RE/100 g) > Sancheong (27.63 mg RE/100 g) > Yeongam (17.99 mg RE/100 g). Our results suggest that persimmon fruits of Hadong may be mainly phenolic compounds when compared to other region samples.
Fig. 3.
Comparison of total phenolic and flavonoid contents in the methanol extracts from persimmon juice harvested from different regions of Korea. 1, Sancheong; 2, Hadong; 3, Sacheon; 4, Jinju; 5, Yeongam; 6, Gurye.
Comparison of organic acid and sugar contents
Previous studies have proved that organic acids and sugars are associated with aroma and the sensation of sweetness in various fruits (8). These two constituents are known to be responsible for maintaining the quality and nutritional value in foods (6). In particular, organic acids have a protective role against various diseases due to their antioxidant activities (28). In this study, the contents of organic acid in persimmon fruit juice were determined by HPLC. Even though the earlier research was measured with three organic acids, including malic acid, citric acid, and fumaric acid (13), ten organic acid compositions were analyzed for this study. As illustrated in Table 1, the highest content was 3712.15 mg/kg in Yeongam, while Hadong showed the lowest content with 2654.72 mg/kg. Differences were observed between individual and total organic acids, and tartaric acid showed the highest differences in all samples. The predominant composition was tartaric acid, followed by malic acid, ascorbic acid, acetic acid, latic acid, citric acid, and oxalic acid, and their average content showed 1876.51, 327.40, 297.21, 198.13, 168.24, 156.34, and 81.58 mg/kg, respectively. However, succinic acid, fumaric acid, and glutaric acid were not detected. Interestingly, malic acid exhibited the highest content (401~1044 mg/kg) in previously reported data, however, tartaric acid was detected the highest content (1396.91~2448.49 mg/kg) in the present study. This phenomenon suggests that the compositions and contents of amino acid in edible plants may be affected by factors including environmental stresses (temperature, light, and area), genetics, cultivars, and agronomic conditions (5).
Table 1.
Comparison of organic acid and sugar content in persimmon juice harvested from different regions of Korea
| Organic acid composition | Organic acid contents (mg/kg)1) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sancheong | Hadong | Sacheon | Jinju | Yeongam | Gurye | |
| Oxalic acid | 68.88 ± 5.87 | 100.27 ± 6.75 | 69.50 ± 7.15 | 58.37 ± 3.95 | 121.16 ± 7.93 | 71.30 ± 3.97 |
| Tartaric acid | 1595.45 ± 92.1 | 1396.91 ± 89.2 | 1960.10 ± 107.3 | 2448.49 ± 117.2 | 2217.44 ± 123.7 | 1640.67 ± 101.5 |
| Malic acid | 316.00 ± 17.5 | 376.25 ± 17.6 | 354.01 ± 21.6 | 198.81 ± 8.72 | 310.95 ± 13.1 | 408.37 ± 14.6 |
| Ascorbic acid | 307.00 ± 15.3 | 323.19 ± 12.9 | 303.14 ± 13.9 | 177.66 ± 8.33 | 356.60 ± 15.7 | 315.67 ± 11.79 |
| Latic acid | 129.28 ± 2.36 | 175.24 ± 9.45 | 132.77 ± 2.88 | 144.04 ± 10.17 | 330.29 ± 12.2 | 97.79 ± 6.38 |
| Acetic acid | 159.13 ± 8.55 | 171.06 ± 9.13 | 214.65 ± 10.27 | 243.36 ± 13.42 | 247.54 ± 8.75 | 153.05 ± 9.27 |
| Citric acid | 127.80 ± 9.37 | 111.80 ± 7.31 | 140.35 ± 10.70 | 203.95 ± 9.68 | 128.18 ± 5.73 | 225.95 ± 123.56 |
| Succinic acid | ND2) | ND | ND | ND | ND | ND |
| Fumaric acid | ND | ND | ND | ND | ND | ND |
| Glutaric acid | ND | ND | ND | ND | ND | ND |
|
| ||||||
| Sugar composition | Sugar contents (g/kg) | |||||
|
| ||||||
| Sancheong | Hadong | Sacheon | Jinju | Yeongam | Gurye | |
|
| ||||||
| Sucrose | 10.25 ± 0.35 | 12.74 ± 0.32 | 8.93 ± 0.45 | 9.13 ± 0.37 | 9.24 ± 0.41 | 11.65 ± 0.42 |
| Fructose | 44.37 ± 1.75 | 47.54 ± 1.93 | 40.99 ± 2.37 | 37.55 ± 1.39 | 41.11 ± 1.29 | 44.92 ± 1.09 |
| Glucose | 62.38 ± 3.69 | 70.32 ± 2.85 | 60.62 ± 3.12 | 55.53 ± 1.95 | 60.87 ± 2.15 | 66.43 ± 1.83 |
All values are presented as the mean ± SD of triplicate determinations.
ND: not detected.
Sugar contents, including sucrose, fructose, and glucose were also determined from the fruit juice of the harvested persimmon in different regions. As shown in Table 1, sugar content ranged from 102.21 to 130.60 g/kg. The highest sugar content was measured in Hadong sample with 130.6 g/kg, whereas the lowest content was observed in Jinju with 102.21 g/kg. Additionally, glucose showed the highest levels with 55.53~70.32 g/kg, followed by fructose (37.55~47.54 g/kg), and sucrose (8.93 ~12.74 g/kg). Even though our results were similar to those of previous studies (13,18), individual and total sugar contents showed higher differences. Therefore, this constituent may be an important factor for the selection process of a high quality species from various regions.
Comparison of phenolic acid and catechin contents
Persimmon fruits are renowned as phenolic compound and carotenoid-rich sources (15) that have been widely used as important remedies because of potential beneficial effects on various chronic diseases (16). Even though numerous studies have analyzed phytochemicals and their biological activities in persimmons (12,15,16, 29), the exact phenolic compounds have still not been fully characterized. For these first attempts at a metabolomics approach of phenolic compounds in persimmon fruit juice, we elucidated nine phenolic acids and seven catechins (Fig. 1) by HPLC analysis. As illustrated in Table 2, the contents of phenolic acid in fruit juice of persimmon were observed in the following order: Hadong (42.27 mg/kg) > Yeongam (39.43 mg/kg) > Jinju (37.15 mg/kg) > Gurye (36.67 mg/kg) > Sacheon (34.72 mg/kg) > Sancheong (34.89 mg/kg). The most abundant phenolic acid was gallic acid (content ranges: 10.83~15.16 mg/kg, average content: 13.06 mg/kg), whereas chorogenic acid, caffeic acid, and p-coumaric acid were not detected in six different region samples. An earlier study found that gallic acid and catechin in fully ripe persimmon fruits made up the predominant compositions and their contents ranged from 1.75 to 24.30 mg/kg in gallic acid and from 3.85 to 19.00 mg/kg in catechin (13). Although there were remarkable differences in catechin contents, our result were similar to that of a previous study, which included the detection of gallic acid (13). In the remaining compositions, ferulic acid was the highest content, followed by vanilic acid, p-hydroxybenzoic acid, protocatechuic acid, and tannic acid, and their average contents were 5.54, 5.22, 4.97, 4.34, and 3.70 mg/kg, respectively (Table 2). It is well-known that phenolic compound contents in various food sources show differences resulting from various factors, including soil type, temperature, water status, genetic, light, and regional characterization (5,30). However, our results showed only slight variations according to the harvested samples. From the results shown above, we conclude that phenolic acid in persimmon fruit juice is not a key factor in the comparison with other nutritional constituents for the selection process of a high quality persimmon. The catechin compositions in fruit juice of persimmon grown in different samples are also shown in Table 2. The highest total catechin content was present in the fruit juice of Hadong with 527.97 mg/kg, followed by Sancheong (515.81 mg/kg), Sacheon (355.29 mg/kg), Gurye (343.39 mg/kg), and Yeongam (261.38 mg/g), whereas Jinju sample showed the lowest content (181.34 mg/kg). In previous studies, epigallocatechin was observed through astringent persimmon (12) and catechin exhibited the predominant content in fully ripe persimmon (13). However, the main catechins found in this study were epicatechin gallate and catechin gallate, and their average content showed 208.99 and 95.51 mg/kg, representing more than 80% of the total average catechin content. Moreover, epicatechin gallate was in greatest concentration in all samples with a range from 106.81~367.05 mg/kg, while gallocatechin gallate showed the lowest concentration (2.37~5.75 mg/kg). Based on the above results, differences of individual and total catechin contents may be affected by factors including agricultural practices, climate factors, and soil types as the results of other natural sources (31,32). Interestingly, the contents of catechin exhibited higher variations than those of phenolic acid in the different samples. Therefore, the results of this work suggest that the catechin contents may be an important factor influencing the anti-oxidant activities in comparison with those of phenolic acids in persimmon fruit juice. In this study, we demonstrated for the first time the variations of catechin content occurring in persimmon fruits of different regions.
Table 2.
Comparison of phenolic acid and catechin contents in persimmon juice harvested from different regions of Korea
| Phenolic acid composition | Phenolic acid contents (mg/kg)1) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sancheong | Hadong | Sacheon | Jinju | Yeongam | Gurye | |
| Gallic acid | 11.80 ± 0.92 | 15.16 ± 0.95 | 13.10 ± 10.25 | 10.83 ± 0.94 | 14.50 ± 0.87 | 12.98 ± 7.45 |
| Protocatechuic acid | 4.02 ± 0.55 | 2.01 ± 2.07 | 4.45 ± 0.79 | 5.59 ± 0.35 | 5.65 ± 0.34 | 4.32 ± 0.52 |
| Tannic acid | 3.16 ± 0.43 | 5.02 ± 0.29 | 3.30 ± 0.71 | 4.28 ± 0.19 | 2.11 ± 0.20 | 4.33 ± 0.39 |
| p-Hydroxybenzoic acid | 4.89 ± 0.81 | 6.27 ± 0.73 | 4.09 ± 0.95 | 5.49 ± 0.53 | 4.67 ± 0.12 | 4.41 ± 0.31 |
| Vanillic acid | 5.27 ± 0.97 | 7.38 ± 0.86 | 4.87 ± 1.03 | 3.11 ± 0.67 | 5.77 ± 0.19 | 4.93 ± 0.26 |
| Chlorogenic acid | ND2) | ND | ND | ND | ND | ND |
| Caffeic acid | ND | ND | ND | ND | ND | ND |
| p-Coumaric acid | ND | ND | 0.98 ± 0.23 | 1.94 ± 0.19 | ND | 1.23 ± 0.10 |
| Ferulic acid | 5.58 ± 0.73 | 6.43 ± 0.25 | 4.10 ± 0.64 | 5.91 ± 0.73 | 6.73 ± 0.07 | 4.47 ± 0.27 |
|
| ||||||
| Catechin composition | Catechin contents (mg/kg) | |||||
|
| ||||||
| Sancheong | Hadong | Sacheon | Jinju | Yeongam | Gurye | |
|
| ||||||
| Epigallocatechin | 11.34 ± 0.72 | 11.19 ± 0.61 | 11.55 ± 0.97 | 10.98 ± 0.47 | 12.77 ± 0.62 | 12.28 ± 0.47 |
| Catechin | 21.39 ± 1.54 | 24.62 ± 1.29 | 23.68 ± 2.11 | 23.84 ± 0.23 | 30.55 ± 2.35 | 25.58 ± 1.75 |
| Epicatechin | 9.15 ± 1.27 | 12.02 ± 0.93 | 7.63 ± 0.39 | 8.15 ± 0.61 | 13.65 ± 0.51 | 12.16 ± 0.41 |
| Epigallocatechin gallate | 9.87 ± 0.79 | 10.60 ± 0.75 | 9.95 ± 0.53 | 5.98 ± 0.35 | 11.32 ± 0.93 | 11.18 ± 0.54 |
| Gallocatechin gallate | 4.51 ± 0.35 | 5.04 ± 0.27 | 2.37 ± 0.13 | 2.84 ± 0.19 | 5.65 ± 0.49 | 5.75 ± 0.36 |
| Epicatechin gallate | 335.15 ± 13.98 | 367.05 ± 12.67 | 186.74 ± 9.76 | 106.81 ± 3.75 | 153.03 ± 0.25 | 176.94 ± 7.35 |
| Catechin gallate | 124.38 ± 5.74 | 97.44 ± 3.82 | 83.37 ± 3.62 | 52.74 ± 1.82 | 70.14 ± 5.77 | 99.00 ± 4.93 |
All values are presented as the mean ± SD of triplicate determinations.
ND: not detected.
Comparison of amino acid content
The quantification of amino acid plays an important role in knowing the nutritional qualities of fruits, vegetables, and foods (7). We evaluated seventeen amino acid components from persimmon fruit juice using HPLC analysis. The total amino acid contents exhibited differences in different samples (Table 3). The persimmon with the most abundant amino acid content levels came from Sacheon (390.75 mg/100 g), followed by Hadong (273.43 mg/100 g), Sancheong (231.18 mg/100 g), Gurye (191.64 mg/100 g), and Jinju (191.39 mg/100 g), while Yeongam showed the lowest content (187.72 mg/100 g). This result suggests that amino acid contents may be also affected by factors including environmental stress (light, moisture, and temperature) and agronomic condition, just as the results of other metabolites in previous studies (5,20). In particular, aspartic acid and glutamic acid exhibited the highest concentration in all samples, at 31.84 and 29.20 mg/100 g, respectively, comprising 13% and 12% of total amino acids (Table 3). Cysteine had the lowest concentration of 0.1% of total average content. In the present study, amino acid showed the highest variations and might be a potential constituent for the quality of persimmon fruits.
Table 3.
Comparison of amino acid contents in persimmon juice harvested from different regions of Korea
| Amino acid composition | Amino acid contents (mg/100 g)1) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sancheong | Hadong | Sacheon | Jinju | Yeongam | Gurye | |
| Aspartic acid | 32.82 ± 2.35 | 34.31 ± 2.76 | 47.55 ± 4.77 | 27.48 ± 1.07 | 25.44 ± 0.83 | 23.45 ± 1.85 |
| Threonine | 16.93 ± 1.57 | 17.54 ± 2.33 | 24.00 ± 2.53 | 12.24 ± 0.63 | 11.14 ± 0.39 | 10.63 ± 0.73 |
| Serine | 14.37 ± 0.98 | 15.43 ± 1.75 | 21.93 ± 3.12 | 12.51 ± 0.54 | 11.27 ± 0.57 | 10.83 ± 0.92 |
| Glutamic acid | 25.86 ± 2.13 | 31.53 ± 2.19 | 56.58 ± 4.29 | 20.75 ± 1.72 | 19.63 ± 1.32 | 20.83 ± 1.35 |
| Proline | 10.88 ± 0.79 | 12.97 ± 0.85 | 23.38 ± 2.11 | 7.23 ± 0.53 | 8.56 ± 0.93 | 7.58 ± 0.36 |
| Glycine | 13.92 ± 1.33 | 15.25 ± 1.28 | 20.59 ± 1.47 | 12.46 ± 0.47 | 13.57 ± 1.16 | 10.24 ± 0.94 |
| Alanine | 14.28 ± 0.95 | 15.86 ± 1.16 | 21.71 ± 1.83 | 12.86 ± 0.38 | 12.39 ± 1.45 | 10.86 ± 0.86 |
| Cysteine | 3.30 ± 0.13 | 2.94 ± 0.23 | 4.62 ± 0.34 | 2.29 ± 0.15 | 2.32 ± 0.46 | 1.95 ± 0.13 |
| Valine | 12.03 ± 0.86 | 13.35 ± 1.57 | 18.87 ± 1.74 | 10.18 ± 0.30 | 10.20 ± 1.24 | 9.64 ± 0.65 |
| Methionine | 9.62 ± 0.71 | 10.84 ± 0.69 | 26.94 ± 1.85 | 8.88 ± 0.43 | 10.99 ± 0.85 | 12.51 ± 0.97 |
| Isoleucine | 7.32 ± 0.47 | 8.15 ± 0.53 | 11.47 ± 0.96 | 6.15 ± 0.54 | 5.70 ± 0.38 | 5.41 ± 0.49 |
| Leucine | 7.31 ± 0.59 | 18.82 ± 0.93 | 6.94 ± 0.73 | 7.13 ± 0.65 | 13.32 ± 1.51 | 7.07 ± 0.85 |
| Tyrosine | 8.56 ± 0.61 | 9.50 ± 0.47 | 14.16 ± 1.70 | 7.19 ± 0.40 | 5.42 ± 0.32 | 4.90 ± 0.67 |
| Phenylalanine | 12.46 ± 1.03 | 13.83 ± 0.35 | 19.63 ± 1.65 | 10.73 ± 0.46 | 9.86 ± 0.71 | 9.08 ± 0.80 |
| Histidine | 5.40 ± 0.24 | 6.31 ± 0.20 | 8.39 ± 0.79 | 4.14 ± 0.31 | 3.95 ± 0.34 | 4.46 ± 0.51 |
| Lysine | 17.98 ± 1.15 | 22.75 ± 1.39 | 33.15 ± 2.53 | 17.48 ± 1.19 | 14.96 ± 1.19 | 16.85 ± 1.56 |
| Arginine | 18.14 ± 1.37 | 24.05 ± 1.78 | 30.84 ± 1.85 | 11.69 ± 0.82 | 9.00 ± 0.42 | 25.35 ± 1.63 |
All values are presented as the mean ± SD of triplicate determinations.
CONCLUSION
The results of the present study documented for the first time the antioxidant activities and nutritional contents in fruit juice of the persimmon harvested from six different regions in Korea. The methanol extract of the persimmon juice showed potent antioxidant effects using four methods of analysis: DPPH radical, ABTS radical, reducing power, and FRAP. Moreover, five nutritional components showed differences between individual and total contents in all samples. The components that exhibited the predominant compositions were tartaric acid, glucose, gallic acid, epicatechin gallate, and aspartic acid. In particular, the fruit juice of Hadong sample displayed the most potent antioxidant activities as well as the highest phenolic acid and catechin contents, while the lowest contents and antioxidant properties were seen with the Jinju persimmons. Consequently, our results suggest that antioxidant properties in persimmon fruits may be dependent on phenolic acid and catechin, including gallic acid and epicatechin gallate. Further studies are needed to identify other phytochemicals and to determine the potential health benefits through their various biological effects.
ACKNOWLEDGMENTS
This work was supported by Farming Corporation Hadong Daeong. Moreover, this work was carried out with the supported of "Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ007457022012)" Rural Development Administration, Republic of Korea.
REFERENCES
- 1.Abeysinghe DC, Li X, Sun CD, Zhang WS, Zhou CH, Chen KS. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. [Google Scholar]
- 2.Deepa N, Kaur C, George B, Singh B, Kapoor HC. Antioxidant constituent in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Sci Technol. 2007;40:121–129. [Google Scholar]
- 3.Lee Y, Cho E, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol. 2007;53:287–292. doi: 10.3177/jnsv.53.287. [DOI] [PubMed] [Google Scholar]
- 4.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. [Google Scholar]
- 5.Hecke K, Herbinger K, Veberic R, Toplak H, Stampar F, Keppel H, Grill D. Sugar-, acid-, and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur J Clin Nutr. 2006;60:1136–1140. doi: 10.1038/sj.ejcn.1602430. [DOI] [PubMed] [Google Scholar]
- 6.Ashoor SH, Knox JM. Determination of organic acids in foods by high-performance liquid chromatography. J Chromatogr. 1982;229:288–292. doi: 10.1016/s0021-9673(01)87727-x. [DOI] [PubMed] [Google Scholar]
- 7.Basarova G, Janousek J. Importance of amino acids in beer technology and quality. Kvasny Prumysl. 2000;46:314–318. [Google Scholar]
- 8.Colaric M, Veberic R, Stampar F, Hudina M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J Sci Food Agric. 2005;85:2611–2616. [Google Scholar]
- 9.Lee BW, Lee JH, Gal SW, Moon YH, Park KH. Selective ABTS radical-scavenging activity of prenylated flavonoids from Cudrania tricuspidata. Biosci Biotechnol Biochem. 2006;70:427–432. doi: 10.1271/bbb.70.427. [DOI] [PubMed] [Google Scholar]
- 10.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- 11.Parr AJ, Bolwell GP. Phenols in plant and in man. The potential for possible nutritional enhancement of this diet by modifying the phenols content or profile. J Sci Food Agric. 2000;80:985–1012. [Google Scholar]
- 12.Suzuki T, Someya S, Hu F, Tanokura M. Comparative study of catechin compositions in five Japanese persimmons (Diospyros kaki) Food Chem. 2005;93:149–152. [Google Scholar]
- 13.Veberic R, Jurhar J, Mikulic-Petkovsek M, Stamper F, Schmitzer V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.) Food Chem. 2010;119:477–483. [Google Scholar]
- 14.An BJ, Bae MJ, Choi C. Chemical structures and isolation of glucosyltransferase inhibitor from the leaves of Korean persimmon. Food Sci Biotechnol. 1998;7:23–27. [Google Scholar]
- 15.George AP, Redpath S. Health and medicinal benefits of persimmon fruit: A review. Adv Hortic Sci. 2008;22:244–249. [Google Scholar]
- 16.Őzen A, Colack A, Dincer B, Gűner S. A diphenolase from persimmon fruits (Diospyros kaki L. Ebenaceae) Food Chem. 2004;85:431–437. [Google Scholar]
- 17.Morales-de la Peña M, Salvia-Trujillo L, Rojas-Graü MA, Martín-Belloso O. Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice-soymilk beverages during refrigerated storage. Food Chem. 2011;129:982–990. doi: 10.1016/j.foodchem.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Candir EE, Ozademir AE, Kaplankiran M, Toplu C. Physico-chemical changes during growth of persimmon fruits in the east Mediterranean region. Sci Hortic. 2009;121:42–48. [Google Scholar]
- 19.Lu Z, He F, Shi Y, Lu M, Yu L. Fermentative product of L(+)-lactic acid using hydrolyzed acorn starch, persimmon juice and wheat bran hydrolysate as nutrients. Bioresource Technol. 2010;101:3642–3648. doi: 10.1016/j.biortech.2009.12.119. [DOI] [PubMed] [Google Scholar]
- 20.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Maksimovic Z, Malencic D, Kovacevic N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresource Technol. 2005;96:873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Duh PD. Antioxidant activity of budrock (Arctium lappa L.): Its scavenging effect on free radical and active oxygen. J Amer Oil Chem Soc. 1998;75:455–461. [Google Scholar]
- 24.Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:117–178. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- 25.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 26.Rice-Evans CA, Miller NJ, Bolwell GP, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 27.Hertog MGL, Hollman PCH, Venema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J Agric Food Chem. 1992;40:1591–1598. [Google Scholar]
- 28.Valentão P, Andrade PB, Rangel J, Ribeiro B, Silva BM, Baptista P, Seabra RM. Effect of the conservation procedure on the contents of phenolic compounds and organic acids in chanterella (Cantharellus cibarius) mushroom. J Agric Food Chem. 2005;53:4925–4931. doi: 10.1021/jf0580263. [DOI] [PubMed] [Google Scholar]
- 29.Paknikar SK, Fondekar KPP, Kirtany JK, Natori S. 4-Hydroxy-5-methylcoumarin derivatives from Diospyros kaki thunb and D. kaki var. sylvestris Makino; structure and synthesis of 11-methylgerberinol. Phytochemistry. 1995;41:931–933. [Google Scholar]
- 30.Prado RDA, Yuste-Rojas M, Sort X, Andrés-Lacueva C, Torres M, Lamuela-Raventós RM. Effect of soil type on wines produced from Vitis vinifera L. cv. Greache in commercial vine yards. J Agric Food Chem. 2007;51:2144–2155. doi: 10.1021/jf062446q. [DOI] [PubMed] [Google Scholar]
- 31.Tekalign T, Hammes PS. Growth and productivity of potato as influenced by cultivar and reproductive growth II. Growth analysis, tuber yield and quality. Sci Hortic. 2005;105:29–44. [Google Scholar]
- 32.Wang L, Yin L, Li D, Zou L, Saito M, Tatsumi E, Li L. Influences of processing and NaCl supplementation on isoflavone contents and composition during douche manufacturing. Food Chem. 2007;101:1247–1253. [Google Scholar]