Abstract
Simian T-cell lymphotropic viruses (STLV), the nonhuman primate counterparts of human T-cell lymphotropic viruses (HTLV), are endemic in many populations of African and Asian monkeys and apes. Although an etiologic link between STLV1 infection and lymphoproliferative disorders such as malignant lymphomas has been suggested in some nonhuman primate species, most STLV infections are inapparent, and infected animals remain clinically healthy. The retroviral transactivator, tax, is well known to increase transcription of viral and cellular genes, resulting in altered cytokine profiles. This study compared the cytokine profiles of peripheral blood mononuclear cell (PBMC) cultures from 25 STLV1-seropositive rhesus macaques (Macaca mulatta) with those of age- and sex-matched seronegative controls. IFNγ, TNFα, IL10, and IL2 levels in unstimulated PBMC culture supernatants were measured at 24, 48, and 72 h by using enzyme immunoassays. IFNγ concentrations were found significantly higher in the supernatants of PBMC cultures of seropositive monkeys as compared with seronegative controls. In addition, although IL2 concentrations were not significantly elevated in the supernatants of PBMC cultures of all seropositive monkeys as compared with all seronegative controls, IL2 levels were increased in a subset of 5 pairs. Increased constitutive cytokine release occurred in the absence of spontaneous proliferation. The increased constitutive release of IFNγ and IL2 suggests that STLV1 alters immune functions in infected but clinically healthy rhesus macaques and further characterizes STLV1 infection of rhesus macaques as a potential model for human HTLV1 infection.
Abbreviations: HTLV, human T cell lymphotropic virus; PBMC, peripheral blood mononuclear cells; STLV, simian T cell lymphotropic virus
Simian T-lymphotropic viruses (STLV) and their human counterparts (HTLV) are collectively referred to as primate T-cell lymphotropic viruses.30,52 STLV type 1 (STLV1) is classified as a type C member of the Deltaretrovirus genus of retroviruses, and is genetically and antigenically closely related to human T-cell lymphotropic virus type 1 (HTLV1).21 STLV1 is endemic in many populations of wild and captive nonhuman primate species indigenous to Africa and Asia, including rhesus macaques (Macaca mulatta).2,11,14,32,38 Published reports of STLV1 seroprevalence in wild populations vary from 0% to 60% depending on the test algorithm used and the species, age, and sex of animals tested.11,17,18,31,32 Estimates of STLV1 seroprevalence in captive populations of macaques housed in facilities in the United States range from 3% to 12%, although hyperendemic populations in which prevalence exceeds 60% have also been identified.11,31,32 The seroprevalence in the general colony at the California National Primate Research Center is 3.7%. Semen, cervical secretions, blood, and breast milk exchanged through sexual, fighting, nursing, or iatrogenic actions have been identified as sources for virus transmission.14,30,52
In humans, infection with HTLV1 is causally linked to the development of adult T-cell leukemia and lymphoma and to a progressive immune-mediated neurologic disease, HTLV1-associated myelopathy–tropical spastic paraparesis.18,26,34 HTLV1 infection has also been associated with other diseases including myositis, uveitis, and infective dermatitis.36,41,55 STLV1 infection has been associated with lymphoproliferative disease and T-cell leukemia–lymphoma in some African primate species, including baboons (Papus spp.), African green monkeys (Chlorocebus spp.), and gorillas (Gorilla spp.).5,10,21,22,28,37,42 To date, no counterpart of HTLV1-associated myelopathy–tropical spastic paraparesis has been recognized in any species of STLV-infected nonhuman primate. The vast majority of STLV infections remain inapparent, and infected primates appear clinically healthy.7,14,30,52 No disease or pathology related to STLV infection has been found in the rhesus macaque colony at our institution; therefore animals for this study were chosen according to their serologic antibody status.
STLV1 and HTLV1 are highly cell-associated viruses, with a predominant cellular tropism for CD4+ and CD8+ T-lymphocytes.15,34,45,46 Both viruses establish persistent, lifelong infections,13,15,34 and replication in vivo involves the clonal expansion of infected T cells.15,23,34 STLV1 and HTLV1 both induce tumor formation in a small percentage of infected subjects, usually after a long period of infection; the respective tumors in humans and nonhuman primates are indistinguishable.52
Although the association of HTLV and STLV infections with overt clinical disease is relatively rare, there are some reports of HTLV1-associated alterations of T-cell surface marker expression, including increased expression of CCR4, CD4, CD25, and HLADR42,45,46,53 and downregulation of the CCR5 and CC chemokines.4 Other HTLV studies, however, have reported normal function of lymphocytes obtained from HTLV-seropositive patients.40 Elevated total leukocyte and absolute lymphocyte counts have been obtained in STLV1-seropositive African green monkeys when compared with seronegative controls.37 We previously reported the absence of significant differences in hematologic parameters, T-cell subsets, and cell-surface marker expression between clinically healthy STLV1-infected rhesus macaques and uninfected age- and sex-matched controls.7 Similarly, the absence of significant differences in T-cell subsets has been reported between clinically healthy STLV1-infected and uninfected mandrills (Mandrillus spp.).45 Previous studies in humans infected with HTLV have noted altered levels of IFNγ, TNFα, IL1a, IL2, IL5, IL6, and IL10.1,8,27,36,42,53 A role for increased levels of inflammatory cytokines, importantly IFNγ, in both the pathology and progression from healthy carriers to HTLV1-associated myelopathy–tropical spastic paraparesis has been proposed.16,19,43 Increased serum levels of IL5 and IL10 are associated with HTLV disease progression and are considered indicators of unfavorable prognosis in patients with HTLV-related adult T-cell leukemia–lymphoma, a peripheral T-cell neoplasm that leads to severe immunocompromise and poor prognosis in most patients.23 Several STLV-transformed cell lines have been found to constitutively release TNFα, granulocyte–macrophage colony-stimulating factor, fibroblast growth factor β, and IL6.29 No significant differences in serum cytokine levels were found when mandrills with naturally acquired STLV infection were compared with uninfected control animals, with the exception of a single STLV-positive mandrill that had significantly elevated levels of IL2, IL6, IL10, IFNγ, and TNFα.46
Spontaneous proliferation of cultured lymphocytes in the absence of stimulation is well documented in humans infected with HTLV1, with as many as 50% of all HTLV-infected patients exhibiting this phenomenon.25,40,46 Reports of spontaneous proliferation of cultured lymphocytes from STLV-infected nonhuman primates are lacking.
The purposes of this study were to investigate differences in cytokine profiles, specifically constitutive cytokine release by unstimulated peripheral blood mononuclear cells (PBMC) in vitro between healthy STLV1-infected rhesus macaques and uninfected controls and to determine whether PBMC obtained from healthy STLV carriers undergo spontaneous proliferation in the absence of stimulation.
Materials and Methods
Study population and specimen procurement.
As in our previous study of phenotypic T-cell subset markers,7 25 STLV1-seropositive rhesus macaques (Macaca mulatta; male: n = 4; age, 7 to 20 y; female: n = 21; age, 2 to 29 y) were identified during routine retroviral screening for SPF colony development and included in this study. The seropositive group included naturally infected macaques both imported and born into the colony. In addition, 25 seronegative macaques, matched for age (± 1 y) and sex, were selected as controls. Routine clinical laboratory data including blood counts and T-cell subset phenotyping collected on colony animals has identified age and sex as significantly affecting normal hematologic and immunologic values (data not shown). All of the macaques were PCR- and antibody-negative for SIV and simian betaretrovirus and seropositive for simian foamy virus, which does not appear to induce IFNγ responses.12 All macaques were anesthetized via intramuscular injection of ketamine hydrochloride (10 mg/kg; Schering-Plough, Union, NJ) after a nonfeeding period of approximately 8 h. Heparinized whole blood was collected from a peripheral vein for culture of PBMC and subsequent cytokine and proliferation assays. Animals were housed indoors and were fed monkey chow (Purina Mills, Richmond, VA) twice daily, with ad libitum access to potable water. Macaques received fruit and vegetable supplements 2 times per week. Trained personnel performed daily morning health checks to evaluate attitude, hydration, appetite, stool, menses, trauma, breathing, weight, hair coat, motor function, and any other abnormal or unusual signs. Any remarkable observations were noted in the animal's health record for appropriate diagnostic or therapeutic follow-up. Macaques were maintained in a fully AAALAC-accredited facility in accordance with the Animal Welfare Act3 and the Guide for the Care and Use of Laboratory Animals.24 All procedures involving animals used in this study were approved by the University of California–Davis IACUC.
Collection and culture of PBMC.
PBMC were separated by density gradient centrifugation (Ficoll Hypaque, ICN Biomedicals INC, Aurora, OH) and resuspended at a concentration of 3× 106 cells per mL in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 20% FBS (Gemini Bio-Products, West Sacramento, CA), 1 mM L-glutamine, and gentamicin (Mediatech, Herndon, VA). The cell suspensions were incubated in a humidified chamber at 37 °C with 5% CO2. At specified time points (24, 48, 72, and 96 h), the cell suspensions were pelleted by centrifugation (1500 × g) and the supernatants removed and stored at −70 °C until assayed.
Cytokine assays.
Levels of IFNγ, TNFα, IL2, and IL10 in culture supernatants were measured by using commercially available enzyme immunoassays for Old World monkeys (U-Cytech, Utrecht, The Netherlands) according to the manufacturers’ instructions. Cytokine standards ranging from 5 to 200 pg/mL were tested with each assay to construct a standard curve for quantitation. These assays and reagents have been validated and optimized for use in rhesus macaques. Briefly, cytokine in supernatant was captured in the antibody-coated wells of microtiter plates. Captured cytokine was bound to a biotinylated detector antibody followed by an enzyme-labeled streptavidin polymer. A chromogenic substrate then was introduced to produce a colored product, which was measured spectrophotometrically.
Proliferation assay.
PBMC cultures from a subset of STLV1-infected macaques (n = 5) and uninfected matched controls (n = 5) were analyzed for evidence of spontaneous proliferation by using flow cytometric analysis of the carboxyfluorescein succinimidyl ester dye dilution method.33 Briefly, PBMC were isolated by density gradient centrifugation, washed, and suspended in RPMI 1640 medium as described previously. Cells were counted, and a subset of the cell population was pelleted and resuspended in prewarmed (37 °C) 1× PBS (Gibco, Carlsbad, CA) containing carboxyfluorescein succinimidyl ester dye (Molecular Probes, Eugene, OR) at a final concentration of 3 μM. After 15 min at 37 °C, dye uptake was stopped by addition of ice-cold (0 °C) RPMI 1640 followed by a 5-min incubation on ice. Cells were washed 2 more times in RPMI 1640 containing 10% FBS to ensure that dye bound to proteins in the supernatant was completely removed, preventing any subsequent uptake into bystander cells. Dye-labeled and unlabeled (control) cells were plated into 96-well plates for experiments and analyses. Mitogen stimulation of PBMC was performed by using concanavalin A (Sigma-Aldrich, St Louis, MO) at a final concentration of 10 µg/mL. Cell viability, when applicable, was assessed by the trypan blue exclusion test47 and flow cytometric analysis using forward- and side-scatter characteristics.
Statistical analyses.
Data analysis was performed by using JMP software (version 10.0.0, SAS, Cary, NC). Normality of the data was assessed by using the Shapiro–Wilk W test. In addition, log- and power transformation and outlier exclusion treatments were applied to the data. Data from each pair (STLV-infected and uninfected) of macaques was treated as a matched pair and analyzed using the Wilcoxon matched-pairs signed rank test for nonparametric distributions. By using Gaussian approximation, the medians of the 2 groups were compared for significance. A P value of 0.05 or less was considered to indicate statistical significance.
Results
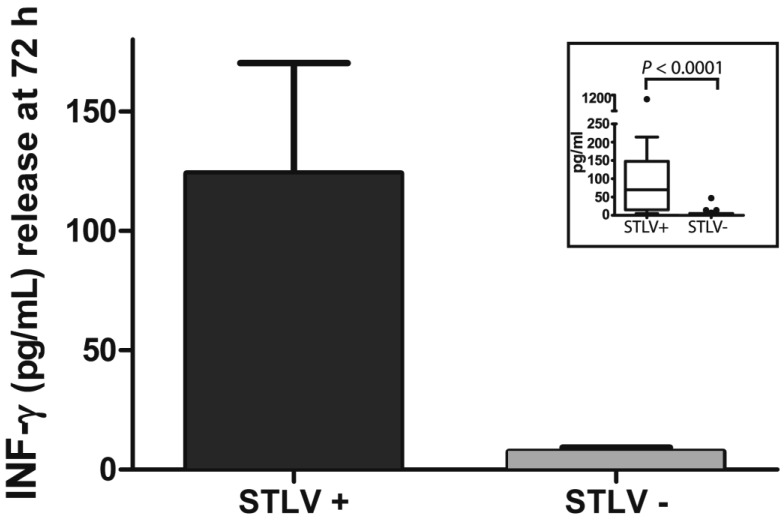
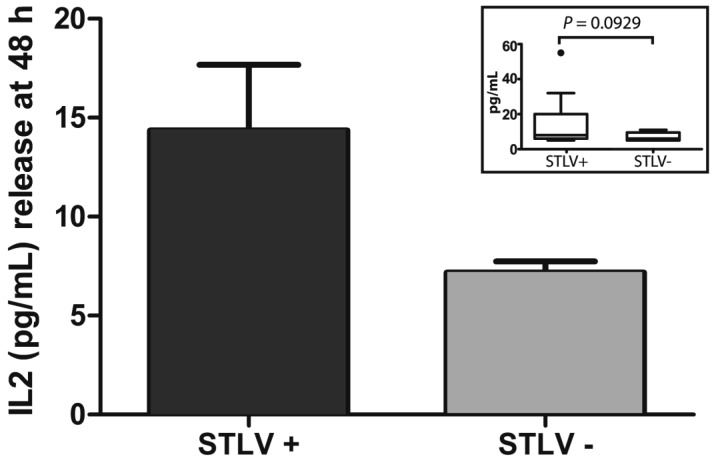
The sex, age, and IFNγ and IL2 levels of our STLV1-infected and uninfected rhesus macaques are shown in Table 1. In preliminary studies, cytokine levels on PBMC from pairs of STLV1-infected and uninfected animals were determined at 24, 48, and 72 h (data not shown). In these pilot studies, the highest levels of IFNγ were seen at 72 h and of IL2 at 48 h; levels of IL10 and TNFα were below the limits of detection (< 5 pg/mL) at all time points analyzed and in both infected and uninfected macaques. Statistical analysis of the data (including log- and power-transformation and outlier exclusion treatments) by using the Shapiro–Wilk W test for goodness of fit demonstrated that the IFNγ and IL2 data were not normally distributed. Therefore, we used the Wilcoxon matched-pairs signed rank test for determining significance. The concentration (mean ± SEM) of IFNγ at 72 h from PBMC of STLV-infected monkeys (Figure 1) was 123.96 ± 46.16 pg/mL compared with 7.48 ± 1.72 pg/mL for uninfected controls. The concentration of IL2 at 48 h in supernatants of STLV1-infected PBMC (Figure 2) was 14.35 ± 3.32 pg/mL compared with 7.18 ± 0.56 pg/mL for uninfected controls. As shown in the insets, the median IFNγ level for STLV-infected monkeys was 70 pg/mL compared with 5 pg/mL for uninfected controls (P < 0.0001); and the median IL2 level for STLV-infected monkeys was 8 pg/mL and 6 pg/mL for uninfected controls (P = 0.0929). In the majority (12 of 17) of animal pairs, the level of IL2 release was low (≤ 12 pg/mL for both groups). However, in the other 5 pairs, cytokine concentrations were elevated in the infected as compared with the uninfected monkey.
Table 1.
Sex, age, and IL2 and IFNγ levels of paired STLV1-infected and -uninfected rhesus macaques
| STLV-infected |
STLV-uninfected |
||||||||
| Macaque | Sex | Age (mo) | IL2 (pg/mL) | IFNγ (pg/mL) | Macaque | Sex | Age (mo) | IL2 (pg/mL) | IFNγ (pg/mL) |
| 17006 | F | 349 | not tested | 43 | 7707 | F | 342 | not tested | <5 |
| 22688 | F | 281 | not tested | 62 | 18485 | F | 276 | not tested | <5 |
| 32223 | F | 257 | <5 | 174 | 32229 | F | 244 | <5 | <5 |
| 32218 | F | 243 | 6 | 140 | 32226 | F | 243 | <5 | <5 |
| 24450 | M | 241 | 7 | <5 | 21876 | M | 232 | 9 | <5 |
| 24453 | M | 231 | not tested | 133 | 21779 | M | 222 | not tested | <5 |
| 32225 | F | 230 | not tested | 158 | 21020 | F | 229 | not tested | <5 |
| 20994 | F | 230 | 6 | 155 | 20976 | F | 230 | 11 | 14 |
| 21225 | F | 227 | 8 | 36 | 21100 | F | 228 | 8 | <5 |
| 32217 | F | 214 | 6 | 32 | 22159 | F | 227 | 10 | <5 |
| 32018 | F | 175 | 20 | 18 | 24375 | F | 183 | 6 | <5 |
| 32023 | F | 174 | 9 | 130 | 24681 | F | 173 | 5 | <5 |
| 32019 | F | 173 | 6 | 214 | 24722 | F | 173 | 10 | 47 |
| 32020 | F | 173 | 20 | <5 | 24676 | F | 173 | 6 | <5 |
| 24640 | M | 163 | not tested | 108 | 24199 | M | 174 | not tested | <5 |
| 32021 | F | 162 | 55 | 1187 | 25568 | F | 160 | <5 | <5 |
| 32033 | F | 139 | 9 | 119 | 26618 | F | 138 | 8 | <5 |
| 33596 | F | 137 | 32 | 162 | 25424 | F | 150 | 5 | <5 |
| 32028 | F | 137 | 8 | 11 | 26679 | F | 137 | 8 | <5 |
| 25928 | F | 134 | not tested | 17 | 25948 | F | 134 | not tested | 14 |
| 31923 | F | 121 | 32 | 92 | 26327 | F | 131 | 5 | <5 |
| 28875 | M | 90 | 6 | 13 | 28717 | M | 91 | 11 | <5 |
| 32032 | F | 83 | not tested | 70 | 28772 | F | 85 | not tested | <5 |
| 32037 | F | 80 | 9 | 10 | 28764 | F | 77 | 5 | <5 |
| 31932 | F | 73 | not tested | <5 | 28982 | F | 84 | not tested | 7 |
Figure 1.
Constitutive IFNγ release (mean ± SE) at 72 h in PBMC from 25 pairs of clinically healthy STLV antibody-positive and -negative rhesus macaques matched for age (± 1 y) and sex. The inset (Tukey boxplot) demonstrates the median and 25th and 75th percentiles of the data. Any individual values greater than the sum of the 75th percentile and the interquartile range (difference between 25th and 75th percentiles) were plotted as outliers (dots). The median values of the 2 groups were significantly (P < 0.0001) different.
Figure 2.
Constitutive IL2 release (mean ± SE) at 48 h in PBMC from 17 pairs of clinically healthy STLV antibody-positive and -negative rhesus macaques matched for age (± 1 y) and sex. The inset (Tukey boxplot) demonstrates the median and 25th and 75th percentiles of the data. Any individual values greater than the sum of the 75th percentile and the interquartile range (difference between 25th and 75th percentiles) were plotted as outliers (dots). The median values of the 2 groups were not significantly different (P = 0.0929).
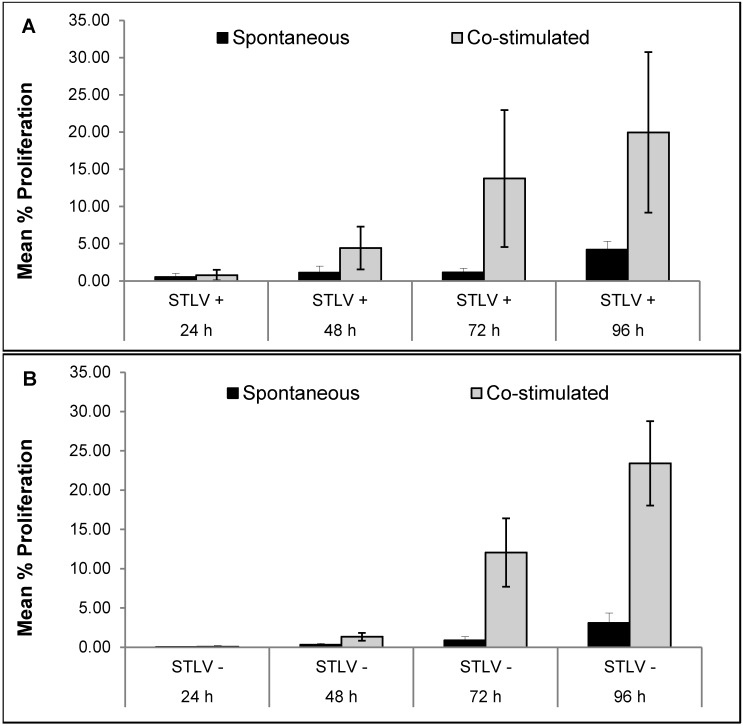
Figure 3 compares the spontaneous and mitogen-induced proliferative responses of PBMC from STLV1-infected and uninfected macaques. During a time course of 96 h, no evidence of spontaneous proliferation was seen in PBMC from STLV1-infected macaques, which constitutively released significantly (P < 0.0001) increased levels of IFNγ. Similarly, cells from uninfected controls showed no spontaneous proliferation during the 96-h observation period. Increased proliferative responses to stimulation with concanavalin A occurred in a time-dependent (24 to 96 h) fashion in PBMC from both infected and uninfected macaques. In addition, the constitutive release of IFNγ from cells of STLV-infected subjects was exacerbated when the same cells were exposed to a reportedly inert dye used in the proliferation assay. No such potentiation of IFNγ release was observed in cells from the uninfected controls.
Figure 3.
Time-dependent proliferative responses in PBMC from 5 pairs of rhesus macaques. Proliferation in mitogen-stimulated and unstimulated cells from (A) STLV-infected macaques and (B) uninfected (STLV-negative) age- and sex-matched controls. Results are expressed as mean ± SEM.
Discussion
The purpose of this study was to determine the effects of STLV infection on the immune system of clinically healthy rhesus macaques. Results clearly demonstrate differences in cytokine profiles of clinically healthy STLV1-infected rhesus macaques compared with uninfected controls. In absence of stimulation, PBMC from asymptomatic STLV-infected rhesus macaques constitutively released significantly higher levels of IFNγ in vitro than did PBMC from STLV1- negative rhesus macaques matched for age and sex. In addition, our data demonstrate that PBMC from a subset of the STLV1-positive macaques produced higher levels of IL2 than did controls. Spontaneous proliferation of PBMC obtained from healthy STLV carriers or their matched controls did not occur in the absence of stimulation.
These findings are consistent with the results of previous human studies demonstrating increased levels of IFNγ and IL2 in the supernatants of lymphocyte cultures from asymptomatic HTLV1 carriers.1,8,19,43,44,49 However, we did not observe abnormally elevated levels of TNFα or IL10 in our macaques, as has been reported in several published studies of HTLV1-infected humans.8,16,19,36,43,44 Increased production of IFNγ and other cytokines has been reported in both asymptomatic HTLV1-infected humans and in those with HTLV-related myelopathy–tropical spastic paraparesis.8,15,16,23,43,54 Some of these reports also suggest that changes in cytokine profiles may be useful for monitoring the development and progression of disease.15,16,36,43 In HTLV-infected humans, high levels of IFNγ may play an important role in the development of parasitic9 or opportunistic44 infections. Other reports suggest that altered cytokine levels produced by HTLV1-positive cells in humans are involved in inflammatory diseases such as uveitis41 and osteoarthritis.55 Although the naturally infected STLV-positive macaques used in the present study constitutively released significantly higher levels of IFNγ than did controls, all of the animals appeared clinically healthy without evidence of secondary infection or other illness reported in their daily health records. Because we were unable to determine the duration of infection for these naturally infected animals, no data were available to establish a correlation between duration (and progression) of infection and cytokine release.
Upregulation of the HTLV tax gene induces substantial increase of expression of the IL2 receptor and secretion of IL2 in HTLV1-infected humans, contributing to the pathogenesis of HTLV1-associated diseases.19,39,49 We hypothesized a similar increased IL2 expression in STLV-infected rhesus macaques. However, we did not observe significant differences in the IL2 levels of the majority (12 of 17) of infected and uninfected animal pairs (IL2 concentration, 12 pg/mL or less in both groups). In this regard, the asymptomatic status of these animals may (directly or indirectly) be related to the lack of significant differences in IL2 levels. Therefore, the increased production of IL2 in the remaining 5 pairs of macaque may be an indication of progressive infection and subsequent development of clinical signs. Recent studies in humans infected with HTLV1 have identified differential cytokine release profiles in T-cell subsets at various time points during the course of infection.19,48,53 Monitoring immune profile changes over time could lead to the discovery of useful signatures associated with disease development and progression. Similar studies investigating the role of various T-cell subsets over time in STLV-infected animals are needed.
In humans, a common response to HTLV1 infection is the development of spontaneous T-cell proliferation.39,49 Both HTLV and STLV can cause and maintain in vitro transformation of susceptible T cells by virus-induced autostimulation of cell proliferation.52 Such transformed cell lines have been shown to constitutively release various cytokines.29 In the current study, we did not find evidence of spontaneous proliferation of STLV1-infected PBMC in the absence of stimulation. Lack of proliferation without stimulation is in agreement with published findings30,35,46 and supports the notion that lymphoproliferative disease is rare and unusual in STLV-infected macaques. However, several groups have reported the occurrence of lymphoproliferative disorders in humans only after prolonged periods of infection.14,30,32 In this regard, perhaps the cohort of STLV-infected macaques we used were at a stage of infection prior to cell transformation. Long-term, longitudinal studies are necessary to address these questions. In addition, such studies of chronic asymptomatic STLV infection in aging macaques could provide a model for emerging studies of immune senescence, the phenotypic and functional changes associated with the aging immune system. Recent publications have demonstrated that chronic immune stimulation from persistent pathogens and the progressive inflammation associated with aging have deleterious effects such as reduced naïve T cell output, altered innate and adaptive effector functions, declining responsiveness to vaccines, increased susceptibility to common pathogens, and reactivation of latent pathogens.6,20
The focus of our study was to evaluate changes in the cytokine release profile of lymphocytes from rhesus macaques naturally infected with STLV1. A wide range of pathogenic and nonpathogenic effects have been observed in various primates infected with different strains of STLV1 typically found in their own or another species.52 For example, T-cell leukemia and lymphoma have been reported in STLV1-infected African green monkeys.42,50 Similarly, cross-species transmission of an STLV1 of rhesus origin resulted in a dramatic increase in the incidence of T-cell leukemia–lymphoma in baboons, a rate much higher than that observed in baboons naturally infected with baboon strains of STLV.51 In addition, transiently increased serum concentrations of IFNγ have occurred in pig-tailed macaques (Macaca nemestrina) during acute infection with the human ACH isolate of HTLV1.35 Each of these virus–host interactions provides valuable insights for understanding the effects of STLV infection on the nonhuman primate immune system, which potentially can serve as a useful model for the study of various aspects of HTLV1 infection of humans.
The altered constitutive release of IFNγ and IL2 in STLV1-infected rhesus macaques suggests possibly deleterious effects of STLV on the immune system of asymptomatic STLV-infected animals. Therefore, interaction between STLV and the immune system and potential changes on immunologic profiles of clinically healthy animals carrying STLV represent potential confounding variables in research protocols that use rhesus macaques of undetermined STLV infection status. A recent publication showed that humans coinfected with HTLV and HIV presented higher levels of Th1 cytokines than did patients infected with either virus alone, suggesting that HTLV1 infection exacerbates the effects of HIV.1 Unnoticed infections with STLV could have a similar effect on experimental protocols that include the use of infectious pathogens such as SIV.
In summary, we have shown that natural STLV infection perturbs normal immune system homeostasis in infected but clinically healthy rhesus macaques. Significantly elevated levels of constitutively released IFNγ from PBMC from STLV-infected macaques were present, but spontaneous proliferation of unstimulated PBMC was absent. Elevated IL2 levels in the STLV-infected members were demonstrated in a subset of 5 pairs of macaques, but the difference did not reach statistical significance for the entire study population. No differences in TNFα or IL10 levels were observed. If a similar nonproliferative status can be demonstrated during disease progression, it could be an important difference between macaques and humans. These potential differences (and similarities) in the immune responses of macaques to STLV infection are relevant in regard to the characterization and use of nonhuman primates as a suitable model of human HTLV infection. With the increasing availability of nonhuman primate-specific immunophenotyping, cytokine and chemokine reagents, and multiplexed techniques, it will be possible to further characterize the cytokines and chemokines released by specific cell subsets, leading to a detailed understanding of how STLV affects various components of the rhesus macaque immune system.
Acknowledgments
This study was supported in part by NCRR/NIH grant number RR00169 to the California National Primate Research Center.
We gratefully acknowledge Dr Peter Barry, who provided expert scientific discussion and review. We thank our current and past colleagues at the Pathogen Detection Laboratory at the California National Primate Research Center at the University of California Davis for contributing their technical expertise and insightful discussion to this work.
References
- 1.Abrahao MH, Lima RG, Netto E, Brites C. 2012. Human lymphotropic virus type 1 coinfection modulates the synthesis of cytokines by peripheral blood mononuclear cells from HIV type 1-infected individuals. AIDS Res Hum Retroviruses 28: 806–808 [DOI] [PubMed] [Google Scholar]
- 2.Andrade MR, Yee J, Barry P, Spinner A, Roberts JA, Cabello PH, Leite JP, Lerche NW. 2003. Prevalence of antibodies to selected viruses in a long-term closed breeding colony of rhesus macaques (Macaca mulatta) in Brazil. Am J Primatol 59:123–128 [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended . 2008. 7 USC §2131–2159. [Google Scholar]
- 4.Barrios CS, Abuerreish M, Lairmore MD, Castillo L, Giam CZ, Beilke MA. 2011. Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CCR5 in human peripheral blood mononuclear cells. Viral Immunol 24:429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakeslee JR, Jr, McClure HM, Anderson DC, Bauer RM, Huff LY, Olsen RG. 1987. Chronic fatal disease in gorillas seropositive for simian T-lymphotropic virus I antibodies. Cancer Lett 37:1–6 [DOI] [PubMed] [Google Scholar]
- 6.Boudet F. 2010. Vaccines for the elderly: the quest for the ideal animal model. J Comp Pathol 142 Suppl 1:S70–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brignolo L, Spinner A, Yee JL, Lerche NW. 2004. Subsets of T cells in healthy rhesus macaques (Macaca mulatta) infected with simian T-lymphotropic virus type 1. Comp Med 54:271–274 [PubMed] [Google Scholar]
- 8.Carvalho EM, Bacellar O, Porto AF, Braga S, Galvao-Castro B, Neva F. 2001. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic virus type 1-infected blood donors. J Acquir Immune Defic Syndr 27:1–6 [DOI] [PubMed] [Google Scholar]
- 9.Carvalho EM, Da Fonseca Porto A. 2004. Epidemiological and clinical interaction between HTLV1 and Strongyloides stercoralis. Parasite Immunol 26:487–497 [DOI] [PubMed] [Google Scholar]
- 10.Cianciolo RE, Butler SD, Eggers JS, Dick EJ, Jr, Leland MM, de la Garza M, Brasky KM, Cummins LB, Hubbard GB. 2007. Spontaneous neoplasia in the baboon (Papio spp.). J Med Primatol 36:61–79 [DOI] [PubMed] [Google Scholar]
- 11.Daniel MD, Letvin NL, Sehgal PK, Schmidt DK, Silva DP, Solomon KR, Hodi FS, Jr, Ringler DJ, Hunt RD, King NW, Desrosiers RC. 1988. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer 41:601–608 [DOI] [PubMed] [Google Scholar]
- 12.Falcone V, Leupold J, Clotten J, Urbanyi E, Herchenroder O, Spatz W, Volk B, Bohm N, Toniolo A, Neumann-Haefelin D, Schweizer M. 1999. Sites of simian foamy virus persistence in naturally infected African green monkeys: latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology 257:7–14 [DOI] [PubMed] [Google Scholar]
- 13.Fletcher MA, Gjerset GF, Hassett J, Donegan E, Parker JW, Mosley JW. 1991. Lymphocyte immunophenotypes among anti-HTLVI/II-positive blood donors and recipients. The Transfusion Safety Study Group. J Acquir Immune Defic Syndr 4:628–632 [PubMed] [Google Scholar]
- 14.Fultz PN. 1992. Simian T-lymphotropic virus type 1 in the Retroviridae. New York (NY): Plenum Press [Google Scholar]
- 15.Funai N, Shimamoto Y, Yoshida S, Nagai Y, Nakazato S, Kohashi O. 1996. Differences in immune functions between human T-lymphotropic virus type I carriers and patients with adult T-cell leukemia–lymphoma. Clin Immunol Immunopathol 80:325–332 [DOI] [PubMed] [Google Scholar]
- 16.Furukawa Y, Saito M, Matsumoto W, Usuku K, Tanaka Y, Izumo S, Osame M. 2003. Different cytokine production in Tax-expressing cells between patients with human T cell lymphotropic virus type I (HTLVI)-associated myelopathy/tropical spastic paraparesis and asymptomatic HTLVI carriers. J Infect Dis 187:1116–1125 [DOI] [PubMed] [Google Scholar]
- 17.Gabet AS, Gessain A, Wattel E. 2003. High simian T-cell leukemia virus type 1 proviral loads combined with genetic stability as a result of cell-associated provirus replication in naturally infected, asymptomatic monkeys. Int J Cancer 107:74–83 [DOI] [PubMed] [Google Scholar]
- 18.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet 2:407–410 [DOI] [PubMed] [Google Scholar]
- 19.Hanon E, Goon P, Taylor GP, Hasegawa H, Tanaka Y, Weber JN, Bangham CR. 2001. High production of interferon γ but not interleukin 2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood 98:721–726 [DOI] [PubMed] [Google Scholar]
- 20.Haynes L, Maue AC. 2009. Effects of aging on T cell function. Curr Opin Immunol 21:414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homma T, Kanki PJ, King NW, Jr, Hunt RD, O'Connell MJ, Letvin NL, Daniel MD, Desrosiers RC, Yang CS, Essex M. 1984. Lymphoma in macaques: association with virus of human T lymphotrophic family. Science 225:716–718 [DOI] [PubMed] [Google Scholar]
- 22.Hubbard GB, Mone JP, Allan JS, Davis KJ, 3rd, Leland MM, Banks PM, Smir B. 1993. Spontaneously generated nonHodgkin's lymphoma in 27 simian T-cell leukemia virus type 1 antibody-positive baboons (Papio species). Lab Anim Sci 43:301–309 [PubMed] [Google Scholar]
- 23.Inagaki A, Ishida T, Ishii T, Komatsu H, Iida S, Ding J, Yonekura K, Takeuchi S, Takatsuka Y, Utsunomiya A, Ueda R. 2006. Clinical significance of serum Th1-, Th2-, and regulatory T cell-associated cytokines in adult T-cell leukemia–lymphoma: high interleukin 5 and 10 levels are significant unfavorable prognostic factors. Int J Cancer 118:3054–3061 [DOI] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 25.Itoyama Y, Minato S, Kira J, Goto I, Sato H, Okochi K, Yamamoto N. 1988. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV1-associated myelopathy. Neurology 38:1302–1307 [DOI] [PubMed] [Google Scholar]
- 26.Johnson JM, Harrod R, Franchini G. 2001. Molecular biology and pathogenesis of the human T-cell leukaemia–lymphotropic virus type 1 (HTLV1). Int J Exp Pathol 82:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal RB, Rudolph DL. 1991. Constitutive production of interleukin 6 and tumor necrosis factor α from spontaneously proliferating T cells in patients with human T-cell lymphotropic virus type 1/2. Blood 78:571–574 [PubMed] [Google Scholar]
- 28.Lapin BA, Iakovleva LA, Voevodin AF, Indzhiia LV, Agrba VZ. 1983. [Study of virus-associated lymphomas in primates using a model of malignant lymphoma of the baboon] Vopr Onkol 29:61–66 [Article in Russian]. [PubMed] [Google Scholar]
- 29.Lazo A, Bailer RT. 1996. Constitutive cytokine release by simian T-cell lymphotrophic virus type I (STLVI) and human T-cell lymphotrophic virus types 1/2 (HTLV1/2) transformed cell lines. J Med Primatol 25:257–266 [DOI] [PubMed] [Google Scholar]
- 30.Lerche NW, Osborn KG. 2003. Simian retrovirus infections: potential confounding variables in primate toxicology studies. Toxicol Pathol 31 Suppl:103–110 [DOI] [PubMed] [Google Scholar]
- 31.Lerche NW, Yee JL, Jennings MB. 1994. Establishing specific retrovirus-free breeding colonies of macaques: an approach to primary screening and surveillance. Lab Anim Sci 44:217–221 [PubMed] [Google Scholar]
- 32.Lowenstine LJ, Pedersen NC, Higgins J, Pallis KC, Uyeda A, Marx P, Lerche NW, Munn RJ, Gardner MB. 1986. Seroepidemiologic survey of captive Old World primates for antibodies to human and simian retroviruses and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer 38:563–574 [DOI] [PubMed] [Google Scholar]
- 33.Lyons AB. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods 243:147–154 [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka M. 2003. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene 22:5131–5140 [DOI] [PubMed] [Google Scholar]
- 35.McGinn TM, Wei Q, Stallworth J, Fultz PN. 2004. Immune responses to HTLV1(ACH) during acute infection of pig-tailed macaques. AIDS Res Hum Retroviruses 20:443–456 [DOI] [PubMed] [Google Scholar]
- 36.Montanheiro PA, Penalva de Oliveira AC, Smid J, Fukumori LM, Olah I, da S Duarte AJ, Casseb J. 2009. The elevated interferon gamma production is an important immunological marker in HAM–TSP pathogenesis. Scand J Immunol 70:403–407 [DOI] [PubMed] [Google Scholar]
- 37.Noda Y, Ishikawa K, Sasagawa A, Honjo S, Mori S, Tsujimoto H, Hayami M. 1986. Hematologic abnormalities similar to the preleukemic state of adult T-cell leukemia in African green monkeys naturally infected with simian T-cell leukemia virus. Jpn J Cancer Res 77:1227–1234 [PubMed] [Google Scholar]
- 38.Otsyula M, Yee J, Jennings M, Suleman M, Gettie A, Tarara R, Isahakia M, Marx P, Lerche N. 1996. Prevalence of antibodies against simian immunodeficiency virus (SIV) and simian T-lymphotropic virus (STLV) in a colony of nonhuman primates in Kenya, East Africa. Ann Trop Med Parasitol 90:65–70 [DOI] [PubMed] [Google Scholar]
- 39.Popovic M, Flomenberg N, Volkman DJ, Mann D, Fauci AS, Dupont B, Gallo RC. 1984. Alteration of T-cell functions by infection with HTLV1 or HTLV2. Science 226:459–462 [DOI] [PubMed] [Google Scholar]
- 40.Prince HE. 1990. American blood donors seropositive for human T-lymphotropic virus types 1/2 exhibit normal lymphocyte subsets. Transfusion 30:787–790 [DOI] [PubMed] [Google Scholar]
- 41.Sagawa K, Mochizuki M, Masuoka K, Katagiri K, Katayama T, Maeda T, Tanimoto A, Sugita S, Watanabe T, Itoh K. 1995. Immunopathological mechanisms of human T cell lymphotropic virus type 1 (HTLVI) uveitis. Detection of HTLVI-infected T cells in the eye and their constitutive cytokine production. J Clin Invest 95:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakakibara I, Sugimoto Y, Sasagawa A, Honjo S, Tsujimoto H, Nakamura H, Hayami M. 1986. Spontaneous malignant lymphoma in an African green monkey naturally infected with simian T-lymphotropic virus (STLV). J Med Primatol 15:311–318 [PubMed] [Google Scholar]
- 43.Santos SB, Porto AF, Muniz AL, de Jesus AR, Magalhaes E, Melo A, Dutra WO, Gollob KJ, Carvalho EM. 2004. Exacerbated inflammatory cellular immune response characteristics of HAM–TSP is observed in a large proportion of HTLV1 asymptomatic carriers. BMC Infect Dis 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimamoto Y, Funai N, Watanabe M, Suga K. 1996. Increased production of interferon γ but not interleukin 4 in human T-lymphotropic virus type 1 carriers. Int J Hematol 64:111–118 [DOI] [PubMed] [Google Scholar]
- 45.Souquiere S, Mouinga-Ondeme A, Makuwa M, Beggio P, Radaelli A, De Giuli Morghen C, Mortreux F, Kazanji M. 2009. T-cell tropism of simian T-cell leukaemia virus type 1 and cytokine profiles in relation to proviral load and immunological changes during chronic infection of naturally infected mandrills (Mandrillus sphinx). J Med Primatol 38:279–289 [DOI] [PubMed] [Google Scholar]
- 46.Souquiere S, Mouinga-Ondeme A, Makuwa M, Hermine O, Kazanji M. 2009. Dynamic interaction between STLV1 proviral load and T-cell response during chronic infection and after immunosuppression in nonhuman primates. PLoS ONE 4:e6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strober W. 2001. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 3 Appendix:3B. [DOI] [PubMed] [Google Scholar]
- 48.Takatsuka N, Hasegawa A, Takamori A, Shimizu Y, Kato H, Ohashi T, Amagasa T, Masuda T, Kannagi M. 2009. Induction of IL10- and IFNγ-producing T-cell responses by autoreactive T-cells expressing human T-cell leukemia virus type 1 Tax. Int Immunol 21:1089–1100 [DOI] [PubMed] [Google Scholar]
- 49.Tendler CL, Greenberg SJ, Blattner WA, Manns A, Murphy E, Fleisher T, Hanchard B, Morgan O, Burton JD, Nelson DL, Waldmann TA. 1990. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type 1-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci USA 87:5218–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsujimoto H, Noda Y, Ishikawa K, Nakamura H, Fukasawa M, Sakakibara I, Sasagawa A, Honjo S, Hayami M. 1987. Development of adult T-cell leukemia-like disease in African green monkey associated with clonal integration of simian T-cell leukemia virus type 1. Cancer Res 47:269–274 [PubMed] [Google Scholar]
- 51.Voevodin A, Samilchuk E, Schatzl H, Boeri E, Franchini G. 1996. Interspecies transmission of macaque simian T-cell leukemia–lymphoma virus type 1 in baboons resulted in an outbreak of malignant lymphoma. J Virol 70:1633–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voevodin AF, Marx PA. 2009. Deltaretroviruses, p 193-216. In: Simian virology. Ames (IA): Wiley–Blackwell [Google Scholar]
- 53.Yamano Y, Araya N, Sato T, Utsunomiya A, Azakami K, Hasegawa D, Izumi T, Fujita H, Aratani S, Yagishita N, Fujii R, Nishioka K, Jacobson S, Nakajima T. 2009. Abnormally high levels of virus-infected IFNγ+ CCR4+ CD4+ CD25+ T cells in a retrovirus-associated neuroinflammatory disorder. PLoS ONE 4:e6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasuda K, Sei Y, Yokoyama MM, Tanaka K, Hara A. 1986. Healthy HTLV1 carriers in Japan: the haematological and immunological characteristics. Br J Haematol 64:195–203 [DOI] [PubMed] [Google Scholar]
- 55.Yoshihara Y, Tsukazaki T, Osaki M, Nakashima M, Hasui K, Shindo H. 2004. Altered expression of inflammatory cytokines in primary osteoarthritis by human T lymphotropic virus type 1 retrovirus infection: a cross-sectional study. Arthritis Res Ther 6:R347–R354 [DOI] [PMC free article] [PubMed] [Google Scholar]