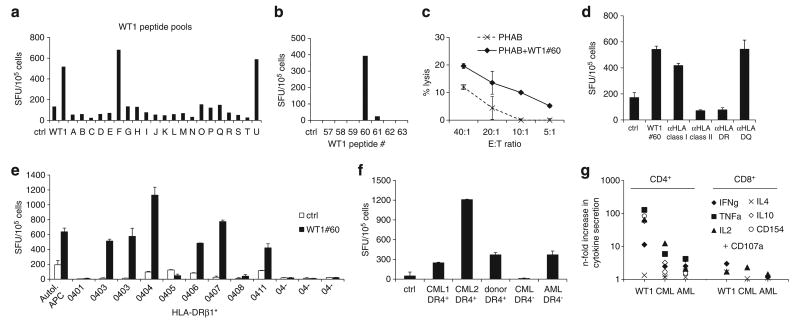
Figure 4.
Characterization of a MHC class II-restricted WT1 epitope. (a) Epitope mapping for WT1 by testing recognition of peptide pools (A–U). Each pool contained 10 or 11 15mer peptides spanning the entire WT1 protein. Recognition of pools F and U indicating recognition of WT1 peptide no. 60, confirmed in (b) by testing WT1 no. 60 and flanking peptides. (c) Specific cytotolytic activity against WT1 no. 60-pulsed autologous PHAB (black line) compared with unpulsed PHAB (dotted line) in a CFSE-based cytotoxicity assay (mean ± s.d.). (d) Recognition of WT1 no. 60 was blocked by preincubation of peptide-pulsed target cells with anti-HLA class II antibody, in particular anti-HLA-DR, indicating a CD4+-restricted recognition of this peptide. (e) Recognition of WT1 no. 60 in IFNγ-ELISpot presented by a variety of HLA-DRβ1*04-positive and -negative antigen-presenting cells (APCs) compared with autologous APCs showing promiscuity in the recognition of WT1 no. 60 for several HLA-DRβ1*04 alleles (mean spot counts ± s.d.). (f) To test for recognition of primary leukemia blasts, CTLs were stimulated with HLA-DRβ1*04-positive and -negative myeloid blast cells and healthy donor PBMC in IFNγ-ELISpot assay (mean ± s.d.) and (g) intracellular cytokine detection; the predominant response was seen mainly in the CD4+ compartment.