FIGURE 6.
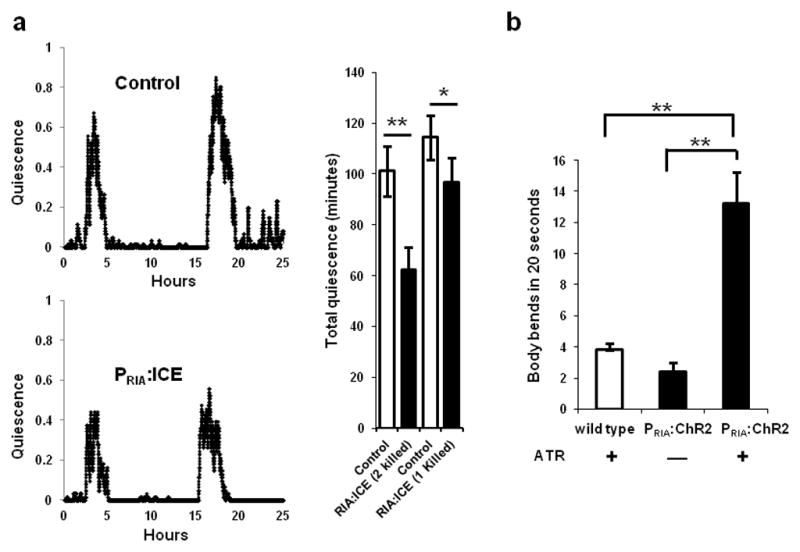
The RIA neurons have both quiescence- and movement-promoting functions. (a) Genetic ablation of the RIA interneurons using the cell death protein ICE (strain:VM1345) decreases quiescence during lethargus compared to control transgenic animals (strain: NQ156). The traces depict the fraction of quiescence in a 10 minute time interval. The top trace is of a control animal of genotype lin-15(n765ts)X; qnEx48[Pins-4:gfp:PEST; lin-15(+)], and the bottom trace is of an experimental animal of genotype lin-15(n765ts)X; akEx211[Pglr-3:gfp; Pglr-3:ICE; lin-15(+)]. Total quiescence during L4 lethargus is lower in experimental animals lacking either one or both RIA neurons than in control animals to which they were paired (*p<0.05, **p<0.001, Student’s two-tailed t-Test, N≥9; Error bars represent s.e.m.). (b) Optogenetic stimulation of the RIA neurons promotes movement during lethargus. WT animals grown on All Trans Retinal (ATR) and PRIA:ChR2 worms grown either in the absence or presence of ATR, were identified as they entered L4 lethargus, transferred to a new plate and were unperturbed for 15 minutes until they became quiescent. Body bends were counted for one minute after a 15-second exposure of quiescent animals to blue light. PRIA:ChR2 animals on ATR moved significantly more than control animals (*p<0.05, **p<0.001, Student’s two-tailed t-Test, WT (N=15), No ATR vs. ATR (N≥20); Error bars represent s.e.m.).
