Abstract
Double-stranded RNA (dsRNA) functions both as a substrate of ADARs and also as a molecular trigger of innate immune responses. ADARs, adenosine deaminases that act on RNA, catalyze the deamination of adenosine (A) to produce inosine (I) in dsRNA. ADARs thereby can destablize RNA structures, because the generated I:U mismatch pairs are less stable than A:U base pairs. Additionally, I is read as G instead of A by ribosomes during translation and by viral RNA-dependent RNA polymerases during RNA replication. Members of several virus families have the capacity to produce dsRNA during viral genome transcription and replication. Sequence changes (A-to-G, and U-to-C) characteristic of A-to-I editing occur during virus growth and persistence. Foreign viral dsRNA also mediates both the induction and the action of interferons. In this chapter our current understanding of the role and significance of ADARs in the context of innate immunity, and as determinants of the outcome of viral infection, will be considered.
1. Introduction
Double-stranded RNA (dsRNA), the substrate of adenosine deaminases acting on RNA (ADARs), also functions as an effector molecule that is sensed by, and modulates the activity of, protein components that mediate the antiviral innate immune response. ADAR activity was described initially in Xenopus oocytes as a dsRNA unwinding activity during antisense RNA studies (Bass and Weintraub 1987; Rebagliati and Melton 1987). But rather than unwinding the dsRNA, it is now known that the deamination of adenosine (A) to inosine (I) that occurred in duplex RNA led to destablization of dsRNA because I:U mismatch bp are less stable than the A:U bp (Bass and Weintraub 1988; Wagner et al. 1989; Serra et al. 2004). Quantitation of the reduced stability of dsRNA following conversion of an A:U bp to a I:U mismatch pair has been described, with an internal I:U pair approximately 2 kcal per mol less stable than an internal A:U pair (Strobel et al. 1994). Because I hydrogen bonds with C instead of U, inosine is decoded as G instead of A by ribosomes during translation and by viral RNA-dependent RNA polymerases during replication and transcription, thereby leading to changes in biologic processes that involve either RNA sequence- or structure-dependent interactions and functions (Bass 2002; Maas et al. 2003; Nishikura 2010; George et al. 2011).
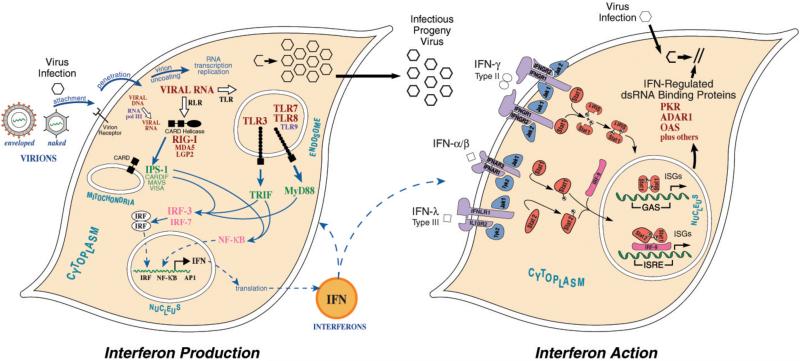
The innate immune response represents an early line of host defense against viral pathogens and includes the induction of interferon, the first cytokine discovered. Interferon (IFN) derives its name from its potent biologic activity, the ability to interfere with virus growth (Isaacs and Lindemann, 1957). Double-stranded RNA (dsRNA) has a long history in the IFN field (Colby, 1971; Stewart, 1979). Both naturally occurring dsRNA in the form of reovirus genome RNA (Tytell et al. 1967) and synthetic polyribonucleotide dsRNA in the form of poly rI:poly rC (Field et al. 1967) were identified over four decades ago as potent inducers of IFN, both in intact animals and in cultured cells. We now have considerable insight into the molecular mechanisms by which dsRNAs are sensed as foreign nucleic acid and activate signal transduction pathways leading to the induction of IFNs and proinflammatory cytokines (Figure 1, left). Among the innate immune signaling sensors that are triggered by pathogen-derived dsRNA leading to the production of IFN are the endosomal toll-like receptor 3, the cytoplasmic RIG-like receptors, RNA polymerase III that acts as a cytoplasmic DNA sensor and produces dsRNAs, and PKR (Uematsu and Akira 2007; Yoneyama and Fujita 2007; O’Neill 2009; Kumar et al. 2011). DsRNAs are important not only as inducers of IFNs, but also as mediators of the actions of IFNs (Samuel 2001, 2007). Much has been learned about the IFN-induced gene products that, acting either alone or in combination with each other, inhibit virus growth in IFN-treated cells (Samuel 2001; Borden et al. 2007). Among these IFN-induced proteins with antiviral activity are two dsRNA-binding proteins that are enzymes activated by dsRNA (Figure 1, right), the protein kinase PKR that phosphorylates translation initiation factor eIF-2 (Samuel 1979, 2001; Sadler and Williams 2007; Pindel and Sadler 2011) and the family of 2’,5’-oligoadenylate synthetases that function via RNase L to degrade RNA (Chakrabarti et al. 2011). A third IFN-induced dsRNA binding protein, the inducible p150 form of ADAR1, utilizes dsRNA as its substrate (Toth et al. 2006; George et al. 2011).
Figure 1.
Signaling pathways activated by RNA leading to interferon production and action in virus-infected cells. (Left) Following entry of virion particles by receptor-mediated fusion or endocytosis and uncoating, the transcription and replication of the viral genome can result in the production of viral RNA with double-stranded character (dsRNA) that is sensed as foreign RNA by pathogen recognition receptors (PRR) of the host. Among the cellular PRR sensors of foreign or non-self viral dsRNA are the RIG-family of cytosolic sensors (RIG-I, MDA5) that act through the mitochondrial adaptor protein IPS-1 (CARDIF, MAVS, VISA) and the endosomal toll-like receptor 3 (TLR3) sensor that acts through the TRIF adaptor protein. SsRNAs are sensed by TLR7 and TLR8, and CpG-rich dsDNA by TLR9, that act through MyD88. Foreign cytosolic dsDNA is also transcribed by RNA polymerase III to generate dsRNA that acts through IPS-1. The IPS-1, TRIF and MyD88 adaptors function downstream of their respective foreign nucleic acid PRRs to signal interferon regulatory factor IRF3 and NF-kB activation and subsequent transcriptional induction of type I interferons. (Right) Interferon proteins initiate signaling by binding to their cognate receptors. The type I interferons that include the IFN-α subspecies and IFN-β act through a shared IFNAR receptor complex to activate the JAK1 and TYK2 protein kinases that lead to the activation of STAT1 and STAT2 signal transducers, which together with IRF9, form the trimeric ISGF3 complex that binds to the 13-bp ISRE DNA element to drive gene transcription. Among the genes induced by JAK-STAT-dependent IFN signaling are those that encode dsRNA-binding proteins including the dsRNA-dependent protein kinase PKR, the family of 2’-5’-oligoadenylate synthetases activated by dsRNA, and the p150 isoform of ADAR1 that acts upon dsRNA. (Adapted from Samuel 2007).
The roles of ADAR proteins in viral infections, both antiviral and proviral, and the function of ADAR1 as a suppressor of innate immune responses including dsRNA-mediated protein activities, are reviewed in this chapter.
2. ADARs and their regulation
2.1 Genes
Three Adar genes have been characterized in mammals; they are designated Adar1, Adar2 and Adar3. Adar1 localizes to human chromosome 1q21 (Wang 1995; Weier et al. 1995) and mouse chromosome 3F2 (Weier et al. 2000), and Adar2 to human chromosome 21q22 (Mittaz et al. 1997) and mouse chromosome 10C1 (Slavov and Gardiner 2002). The Adar1 and Adar2 genes encode active deaminase enzymes that possess A-to-I RNA editing activity, whereas Adar3 does not show demonstrable enzymatic activity (Bass 2002; Maas et al. 2003; Toth et al. 2006; Nishikura 2010; Samuel 2011). The Adar1 gene transcripts possess 15 exons in the human (Liu et al. 1997) and the mouse (Hartner et al. 2004; Wang et al. 2004; George et al. 2005). Both the human and mouse Adar2 genes also have 15 exons (Slavov and Gardiner 2002; Maas and Gommans 2009). Furthermore, multiple splice variants of both Adar1 and Adar2 transcripts have been described, in addition to a complex arrangement of multiple promoters that drive the expression of the Adar1 gene (George et al. 2011).
A single major Adar1 transcript of ~6.7-kb is detected by Northern analysis with RNA from human cells using probes derived from exons 2 to 15 (Patterson et al. 1995). While inducible by interferon and also following infection, a significant basal expression level of Adar1 is observed both in cultured mammalian cells and animal tissues (Patterson et al. 1995; Shtrichman et al. 2002; George et al. 2005). The transcription of the Adar1 gene occurs from at least three promoters, one of which is IFN inducible, in human (George and Samuel, 1999a, 1999b; Kawakubo and Samuel 2000) and mouse (George et al. 2005, 2008) cell lines. Adar1 gene promoters drive the expression of transcripts with alternative exon 1 structures (exon 1A, 1B, 1C) that are spliced as far as is known to a common exon 2 junction. In addition to the three different forms of exon 1, splice variants of other exons, notably exon 7, are also observed (Toth et al. 2006; George et al. 2011).
Among the best-characterized transcriptional units are the two major alternative promoters responsible for the expression of two differently sized isoforms of ADAR1 protein, known generally as p110 and p150. The IFN inducible promoter drives transcription beginning with exon 1A that possesses a translation initiation codon (AUG1) and typically includes the alternative “b” form of exon 7 (George and Samuel 1999a,b; George et al. 2005). The major constitutively active promoter drives transcription beginning with exon 1B and includes the alternative “a” form of exon 7 that is 26 amino acids longer than exon 7b (Liu et al. 1997; George and Samuel 1999a,b; George et al. 2005). Translation initiation of the exon 1B-containing constitutively expressed transcripts begins at AUG296 within the unusually large exon 2. While the consensus open reading frame of the ADAR1 human cDNA is 1226 amino acids (Kim et al. 1994; O’Connell et al. 1995; Patterson and Samuel 1995), because of the alternative splicing involving exons 1 and 7, multiple unique transcripts are produced of ~6.7-kb. The inducible exon 1A-containing transcript with 7b encodes the IFN-inducible p150 protein that is deduced to be 1200 amino acids in size, whereas the constitutively expressed human p110 protein encoded by exon 1B-containing mRNA is deduced to be 931amino acids (Toth et al. 2006; George et al. 2011).
The interferon-inducible Adar1 gene promoter region possesses a consensus interferon-stimulated response element (ISRE). The Adar1 ISRE is highly conserved between the inducible human (George et al. 1999b) and mouse (George et al. 2008) promoter sequences as well as with the ISRE elements present in the IFN inducible promoter of the mouse and human RNA-dependent protein kinase Pkr genes (Tanaka and Samuel 1994; Kuhen and Samuel 1997). The Adar1 human and mouse gene ISRE elements are 12-bp and differ in only one position between each other, at the 3’-position which is either a T or C (GGAAA_CGAAAGT/C). The Pkr human and mouse gene ISRE elements are 13-bp and likewise differ in only the 3’ position from each other (GGAAAACGAAACT/A). ISRE elements enhance transcription in response to type I IFN treatment (Schindler et al. 2007; Randall and Goodbourn 2008). The IFN inducible Adar1 promoter (George et al. 1999b, 2008), like the IFN inducible Pkr promoter (Tanaka and Samuel 1994; Kuhen and Samuel 1997), is TATA-less both in mouse and human cells. Transcriptional activation of genes by type I IFNs involve binding of the IFN (α/β) to the IFNAR receptor. Subsequent activation of JAK1 and TYK2 tyrosine kinases mediate activation of the STAT1 and STAT2 factors that together with IRF9 form the heterotrimeric ISGF3 complex that binds to the ISRE element to enhance gene expression (Figure 1, right). As expected, IFN induction of the Adar1-encoded p150 protein is impaired in mouse embryo fibroblasts that are genetically deficient in either the IFNAR receptor, the JAK1 kinase or STAT2 (George et al. 2008). But, unexpectedly, IFN induction of p150 is independent of STAT1 in MEFs (George et al. 2008). The mechanism of STAT1-independent IFN induction of p150 ADAR1 is not established. Interestingly the DNA editing enzyme APOBEC3G that catalyzes C-to-U deamination of first-strand retrovirus DNA likewise is reported to be induced in a STAT1-independent manner (Sarkis et al. 2006).
2.2 Proteins
The ADAR proteins possess multiple biochemical activities that include the ability to bind A-form dsRNA, Z-form dsDNA and Z-form dsRNA, and to deaminate adenosine in RNA substrates either in a highly selective manner at only one or a very few adenosines, or when the dsRNA substrate possesses extensive duplex character, then at multiple adenosine sites in a non-selective manner (Bass 2002; Maas et al. 2003; Toth et al. 2006; Nishikura 2010; George et al. 2011). The diversity of Adar1 and Adar2 transcripts with alternative exon structures suggests the existence of different ADAR1 and ADAR2 protein isoforms with potentially different activities that might include different substrate specificities, different subcellular localizations, and different capacities to interact with protein partners or bind different nucleic acids. Among the best-characterized ADAR1 protein differences are those that arise from the alternative promoter usage and alternative splicing to generate the two size isoforms of ADAR1. The large or p150 form of ADAR1 is IFN inducible and localizes to both the cytoplasm and nucleus (Patterson and Samuel 1995; Poulsen et al. 2001; Strehblow et al. 2002). The small or p110 form of ADAR1 is constitutively expressed and is predominantly if not exclusively found in the nucleus (Patterson and Samuel 1995; Li et al. 2010). ADAR2 likewise is constitutively expressed and localizes exclusively to the nucleus, primarily in nucleoli (Desterro et al 2003; Sansam et al. 2003). The catalytic and nucleic acid binding domain organization and function of the ADAR proteins are considered in depth elsewhere in this volume by Beal and Allain and their colleagues (Goodman et al. 2011; Barraud and Allain, 2011). Briefly, for both the p110 and p150 isoforms of ADAR1, the deaminase catalytic domain is present in the C-terminal region and both size isoforms possess three copies of the dsRNA-binding motif (R, or dsRBD) positioned in the central region of p110 and p150 (Kim et al. 1994; O’Connell et al. 1995; Patterson and Samuel 1995; Liu and Samuel 1996; Liu et al. 1997). The dsRBD motifs of ADARs including ADAR1 are similar to the prototype dsRNA-binding motif first identified in PKR (McCormick et al. 1992,1995; Fierro-Monti and Mathews 2000). The p150 protein, however, has an N-terminal extension of 295 amino acids compared to p110 ADAR1. The N-terminus of p150 includes a repeated domain (Zα, Zβ) with homology to a sequence present within the N-terminal half of the poxvirus E3L protein (Patterson and Samuel 1995) and this is now known as the Z-DNA binding domain (Herbert et al. 1997; Schwartz et al. 1999). Only one copy of the domain (Zβ) is present in p110. Only more recently have insights been gained regarding the possible physiologic function of the Z-domains present in ADAR1. Interestingly, the Zα domain of ADAR1 p150 was shown to bind Z-RNA in addition to Z-DNA (Brown et al. 2000; Placido et al. 2007). The Zα domain of ADAR1 also forms stable complexes with ribosomes with high affinity in human cells, and a stoichiometric association blocks translation (Feng et al. 2011).
3. Innate immunity signal transduction pathways
3.1 Sensors and adaptors of dsRNA
Viral nucleic acids produced in infected cells include double-stranded and 5’-triphospate-containing RNAs that are sensed as foreign or non-self nucleic acids, thereby triggering the innate immune response (Yoneyama and Fujita 2007, 2010; O’Neill 2009; Kumar et al. 2011; Nakhaei et al. 2009; Kawai and Akira 2010; Garcia-Sastre 2011). 5’-Capping and 2’-O methylation of RNAs, modifications characteristic of self or host mRNAs, generally prevent recognition by host cell cytoplasmic sensors. Among the cellular pattern recognition receptors (PRRs) that sense and respond to foreign viral RNAs are the cytoplasmic retinoic acid-inducible gene I (RIG-I) –like receptors (RLRs) and the membrane-associated Toll-like receptors (TLRs) as illustrated in Figure 1 left. DsRNA is sensed by endosomal TLR3, whereas endosome-localized TLRs 7 and 8 detect single-stranded RNA and TLR9 detects CpG-rich unmethylated DNA. DsRNA also is sensed by RIG-I and MDA5 RLRs, with both the size of A-form dsRNA and the 5’-end structure of the RNA serving as contributing determinants for signaling via RIG-I or MDA-5 which are not functionally redundant. RLR signaling occurs via the mitochondrial IPS-1 adaptor, leading to the activation of IRF3 and NF-κB transcription factors, whereas TLR3 uses the TRIF adaptor, and TLR 7, 8 and 9 use the MyD88 adaptor. Cytoplasmic pathogen B-form DNA can be sensed by RNA pol III among other sensors, which gives rise to RNA subsequently detected by the RLR pathway.
Finally, the RNA-dependent protein kinase PKR functions also as a sensor of dsRNA in a manner that modulates the innate immune response leading to IFN production. Among the viruses where PKR plays a role in the induction of IFN are measles virus, human parainfluenza virus (HPIV) and rotavirus. PKR enhances the induction of IFN-β by measles virus C and V deletion mutants by a pathway dependent upon the IPS-1 adaptor of the RLRs but not TRIF (McAllister et al. 2010). PKR activation also contributes to IFN-β induction via RLR signaling that follows the kinetics of dsRNA accumulation in human parainfluenza virus-infected cells (Boonyaratanakornkit et al. 2011), and the induction and secretion of IFN-β by rotavirus is likewise dependent upon PKR in addition to IPS-1and IRF3 (Sen et al. 2011).
3.2 Suppression of interferon responses by ADARs
A combination of findings from different lines of experimentation are consistent with the notion that ADAR1 functions as a suppressor of interferon responses. Studies including inducible Adar1 gene disruption in mice (Hartner et al. 2009), stable knockdown of both p110 and p150 ADAR1 proteins in human cells in culture (Toth et al. 2009; Li et al. 2010), and transfection analyses with defined I:U-dsRNAs corresponding to hyperedited dsRNAs compared to control RNAs (Vitali and Scadden 2010) taken together suggest that ADAR1 acts as a suppressor of IFN system responses. Given that poly(rI):poly(rC) is both a potent inducer of IFN (Stewart 1979; Yonemaya and Fujita 2007) and an activator of IFN-induced dsRNA-dependent enzymes including PKR and 2’,5’OAS (Samuel 2001), the finding that ADAR1 behaved as a suppressor of IFN responses was somewhat unexpected.
The inducible genetic disruption of Adar1 p110 and p150 expression in mice suggested that ADAR1 functions a suppressor of IFN signaling in addition to displaying an essential role for maintenance of both fetal and adult hematopoietic stem cells (Hartner et al. 2009). Loss of ADAR1 in hematopoietic stem cells led to rapid apoptosis and a global IFN response characterized by an upregulation of IFN-inducible transcripts as revealed by genome-wide transcriptome analyses. The gene expression signature of Adar1−/− cells showed similarities with the signatures of IFN-treated or virus-infected cells. Among the transcripts enhanced by ADAR1 deficiency were those for the PKR, RIG-I and TLR3, all of which are dsRNA sensors (Uemitsu and Akira 2007; Yonemaya and Fujita 2007; Samuel 2001), in addition to STAT1 and 2, IRF 1, 7 and 9, and Mx (Hartner et al. 2009). Human HeLa cells in culture made stably deficient in the expression of ADAR1 p110 and p150 proteins through the use of a short hairpin RNA-mediated knockdown strategy also revealed that apoptosis was enhanced in the ADAR1-deficient cells following infection with wild-type and V-deletion mutant measles virus (Toth et al. 2009). Furthermore, the level of PKR kinase activation and eIF-2α phosphorylation was increased both in HeLa cells stably knocked down for ADAR1 (Li et al. 2010) and 293T cells transiently knocked down for ADAR1 (Nie et al. 2007). The enhanced apoptosis seen in the ADAR1-deficient cells following measles virus infection correlated with enhanced activation of the PKR kinase and IRF3 transcription factor, both key dsRNA sensors in the IFN system. These results suggest that the anti-apoptotic activity of ADAR1 in measles virus-infected HeLa cells is achieved through suppression of pro-apoptotic and dsRNA-dependent activities illustrated by PKR and IRF3 (Toth et al. 2009). Likewise, MEF cells genetically deficient for the p150 isoform of ADAR1 and stably expressing the measles virus receptor displayed extensive virus-induced cytopathic effects following measles virus infection compared to wild-type MEF cells (Ward et al. 2011).
The mechanism by which ADAR1 modulates the expression of IFN inducible genes (characterized by elevated ISG expression in the absence of ADAR1) and the activation of dsRNA-dependent IFN system proteins (illustrated by the elevated activation both of the IFN inducible PKR kinase and the IRF3 transcription factor in the absence of ADAR1) is not yet fully elucidated. One possibility is that ADAR1 functions as a gatekeeper and modifies the structure of activator RNAs and, that when they are edited, the RNAs do not possess the structure sufficient to trigger the signaling and activation of the IFN response. But in the absence of ADAR, the integrity and amount of the structured RNA allows for induction and activation of the IFN system. Another possibility is that the A-to-I editing activity per se of ADAR1 is not required, but rather protein or nucleic acid binding interactions involving ADAR1 as a binding partner are sufficient, and these interactions become altered in the absence of ADAR1. I:U dsRNA, a mimic of hyperedited dsRNA, when transfected into HeLa cells suppresses the induction of IFN inducible genes and apoptosis by poly(rI);poly(rC) compared to control dsRNA (Vitali and Scadden, 2010). Furthermore, transfection of I:U-dsRNA inhibited activation of IRF3 in parental HeLa cells (Vitali and Scadden, 2010), consistent with the enhanced activation of IRF3 and increased apoptosis earlier seen in ADAR1 deficient HeLa cells following infection (Toth et al. 2009). As earlier mentioned, the PKR enhancement of IFN-β induction by virus infection requires the IPS-1 adaptor of the RIG-I / MDA5 signaling pathway that leads to IRF3 factor activation and subsequently to IFN induction (McAllister et al 2010; Boonyaratanakornkit et al. 2011; Sen et al. 2011). That ADAR1 deficiency increases PKR activation (Toth et al. 2009; Li et al. 2010) and that I:U-dsRNA binds both RIG-I and MDA5 in a manner that may interfere with their sensing of foreign dsRNA (Vitali and Scadden 2010) suggests that multiple mechanisms may contribute to the ADAR1-mediated modulation of IRF3 activation status and apoptosis induction.
4. ADARs and their effects on virus-host interactions
The profound importance of ADARs for normal development and neurophysiology in the absence of virus infection is illustrated in mice and flies by the phenotypes observed following genetic disruption of Adar genes or overexpression of ADAR proteins as reviewed by Hartner and by Keegan and colleagues in other chapters in this volume (Hartner and Walkley 2011; Paro et al. 2011). Genetic knockout of Adar1 in the mouse in a manner that disrupts expression of both p110 and p150 (Hartner et al. 2004, 2009; Wang et al. 2004; XuFeng et al. 2009) or only p150 (Ward et al. 2011) results in embryonic lethality between embryonic days 11.5 and 12.5. Genetic knockout of Adar2 in the mouse, while not embryonic lethal, results in neurophysiological abnormalities (Higuchi et al. 2000), whereas overexpression of ADAR2 protein results in adult-onset obesity (Singh et al. 2007).
A number of studies also have revealed the importance of ADAR proteins and A-to-I editing during viral infections (Table 1). Somewhat surprising, the effect of ADARs on the virus-host interaction can be either antiviral or proviral, dependent upon the specific animal virus and mammalian host cell combination and the level and type of ADAR protein expression. The multiple roles played by ADARs will be illustrated in the following sections by considering examples of animal viruses from families with different genome organizations and subcellular sites of replication. These include viruses that have single-stranded RNA genomes of either negative, positive or ambisense coding organization; double-stranded RNA genomes; and double-stranded DNA genomes (Knipe et al. 2007). Present understanding suggests that ADARs may act either directly on the virus by editing a viral RNA in a manner that impacts the outcome of the viral infection, or conceivably indirectly by editing a cellular RNA in a manner that alters a cellular product that subsequently impacts the interaction of the virus with the host. It is also possible that ADARs may function in an editing independent manner, by altering protein or nucleic acid binding interactions, that subsequently affect the outcome of the viral infection. ADAR1 and ADAR2 have overlapping specificities with some substrates (Lehmann and Bass, 2000). In those instances where the effect of ADAR protein function is exerted directly on the viral nucleic acid as implicated by viral sequence changes characterized by A-to-G (or U-to-C) substitutions, for viruses with exclusively a cytoplasmic localization for their multiplication, presumably the p150 isoform of ADAR1 would be the ADAR protein likely responsible; p150 ADAR1 is the only known cytoplasmic ADAR in mammalian cells (Bass 2002; Toth et al. 2006; Nishikura 2010; George et al. 2011). By contrast, for those viruses with a nuclear component to their multiplication that display A-to-G and U-to-C substitutions in the viral sequences, viruses which include dsDNA viruses and orthomyxoviruses among those considered below, then either ADAR1 size isoform or ADAR2 theoretically could be responsible for the editing events as all of these ADARs are nuclear proteins and enzymatically active deaminases (Bass 2002; Toth et al. 2006; Nishikura 2010; George et al. 2011).
Table 1.
Functions of ADARs in viral infections can be either anti- or pro- viral dependent upon the virus-host combination
| Viral Genome Form and Virus | Effect attributed to ADAR | Reference* |
|---|---|---|
| Negative-stranded RNA | ||
| measles virus | U->C,A->G; M RNA | Cattaneo et al.1986,1988; |
| Wong et al. 1991 | ||
| Schmid et al.1992 | ||
| Baczko et al.1993 | ||
| A->G; M RNA | Suspène et al. 2008, 2011 | |
| Protection against CPE; inhibition of PKR and IRF3 activation |
Toth et al.2009 | |
| Antiviral; protection against CPE | Ward et al.2011 | |
| respiratory syncytial virus | A->G; gp G RNA | Rueda et al.1994; Marínez and Melero 2002 |
| parainfluenza virus 3 | RNA 3’-region | Murphy et al.1991 |
| Newcastle disease virus, canine distemper virus, Sendai virus |
Protection against CPE | Ward et al.2011 |
| influenza virus | A->G, M1 RNA | tenOever et al.2007 |
| A->G, HA RNA | Suspène et al.2011 | |
| Protection against CPE | Ward et al.2011 | |
| vesicular stomatitis virus | A->G,U->C | O’Hara et al.1984 |
| Proviral; inhibition of PKR activation | Nie et al.2007 | |
| No effect; inhibition of PKR activation | Li et al.2010; Ward et al. 2010 | |
| Rift Valley fever virus | A->G; L RNA | Suspène et al.2008 |
| lymphocytic choriomeningitis v. | A->G,U->C | Grande-Pèrez et al.2002 |
| A->G,U->C; GP RNA | Zahn et al.2007 | |
| No effect | Ward et al.2011 | |
| hepatitis delta virus | Proviral; selective A->G, amber->W | Taylor, 2003; Casey, 2011 |
| Positive-stranded RNA | ||
| hepatitis C virus | Antiviral | Taylor et al.2005 |
| Double-stranded RNA | ||
| orthoreovirus | No effect | Ward et al.2011. |
| Retroviruses | ||
| human immunodeficiency virus 1 | A->G TAR RNA | Sharmeen et al.1991 |
| Proviral; A->G RRE | Phuphukrat et al.2008 | |
| Proviral; inhibition of PKR activation | Clerzius et al.2009; Doria et al.2009,2011 | |
| avian viruses: ALV and RAV−1 | A->G | Felder et al.1994; Hajjar andLinial, 1995 |
| Double-stranded DNA | ||
| polyoma virus | A->G early/late RNA overlap | Kumar and Carmichael1997; Gu et al.2009 |
| Kaposi sarcoma-associated virus | Selective A->G K12 RNA | Gandy et al.2007 |
| Epstein-Barr virus | A->G BART6 miR | Iizasa et al.2010 |
See text for additional references.
4.1 Negative-stranded RNA viruses
Negative-stranded RNA viruses include those viruses with a single-stranded RNA genome that is the opposite sense, or complementary, to that of mRNA, and hence is not decoded directly by translation. Following infection, the viral genome of negative-stranded ssRNA viruses is transcribed by a virion-associated viral RNA-dependent RNA polymerase to produce mRNA for translation, and then replicated to produce full-length positive (and subsequently negative) sense ssRNA. Among the negative-stranded viruses are members of the Paramyxoviridae, Orthomyxoviridae, and Rhabdoviridae families. The observations of A-to-G (U-to-C) nucleotide substitutions in viral RNA sequences determined for negative-stranded RNA viruses has a long history and has implicated ADAR-mediated A-to-I editing as factor in the interaction of these viruses with their hosts (Cattaneo and Billeter 1992; Samuel, 2011). Only more recently has evidence been obtained that directly establishes ADAR1 as a restriction factor affecting the outcome of interaction of negative-stranded viruses, particularly measles virus, with their hosts (Toth et al. 2009; Ward et al. 2011).
Paramyxoviridae and Orthomyxoviridae
Measles virus (MV) is a member of the Morbillivirus genus of Paramyxoviridae. The enveloped virion of MV encloses a ~16-kb negative-stranded ssRNA genome that consists of six genes, the N, M, F, H and L monocistronic genes and the polycistronic P/V/C gene. The co-transcriptional pseudo-templated G nucleotide insertion editing that occurs with measles virus to generate the V transcript with a frameshift compared to the P/C transcript, which is commonly referred to as “editing” by virologists, should not be confused with the nucleotide substitution editing by ADARs. While measles virus causes a typically acute infection spread by the respiratory route, a rare but serious complication is the subsequent persistent infection of the central nervous system known as subacute schlerosing panencephalitis (SSPE), a progressive fatal neurodegenerative disease (Moss and Griffin 2006; Oldstone 2009). It is in the context of SSPE where ADAR was initially implicated as causing hypermutations characterized by A-to-G substitutions (or U-to-C in the complementary strand). Measles virus isolated from the central nervous system of SSPE patients differed from wild-type MV of acute infections. The novel genetic changes of the SSPE virus were characterized by extensive mutations affecting predominantly the MV matrix M gene and the fusion F gene (Cattaneo and Billeter 1992; Cattaneo et al. 1986; Wong et al. 1991, 1994; Schmid et al. 1992; Baczko et al. 1993). Most striking were biased hypermutations, primarily in the M gene where in one instance up to 50% of the U residues were converted to C. The observed M gene mutations are consistent with the defective production of viral matrix protein associated with SSPE persistent infection in the brain, and the lack of antibodies to M protein seen in SSPE patients (Hall et al. 1979; Liebert et al. 1986). Direct evidence that the hypermutated M gene of a SSPE strain virus contributes to the persistent infection and chronic CNS disease was obtained in studies with transgenic CD46 mice (Oldstone et al. 1999) and the Edmonston strain MV that causes acute infection, which was engineered by reverse genetics to replace the Edmonston M gene with the Biken SSPE strain M gene (Patterson et al. 2001).
Evidence for the editing of measles virus RNA in cell culture infections was obtained using a creative PCR-based strategy (3DI-PCR) to selectively amplify GC-rich RNAs, as would arise following ADAR editing which substitutes I(G ) for A (or C for U in the complementary strand). With the 3DI-PCR approach the natural hydrogen-bonding rule is inversed by generating modified DNA that contains diaminopurine in place of adenine, and inosine in place of guanine, which allows for the selective amplification of GC-rich ADAR-edited RNAs by differential DNA denaturation. When the Schwarz strain of MV was grown on IFN sensitive MRC5 cells, MV sequences were obtained following 3DI-PCR amplification that had extensive A-to-G transitions characteristic of ADAR editing, whereas sequences amplified from MV grown on Vero cells that do not produce type I IFN α or β (and hence would not produce the induced p150 ADAR1) did not show the A-to-G transitions (Suspéne et al. 2008). Using the 3DI-PCR approach it also was shown that a region of the M gene of the live attenuated measles vaccine virus and a region of the HA gene of inactivated seasonal influenza virus vaccine both possessed hypermutated sequences, with the mutation frequency greater for influenza compared to measles virus (Suspéne et al. 2011). ADAR1 p150 earlier was implicated to edit influenza virus RNA, as A-to-G mutations were frequently found in influenza virus matrix M1 RNA isolated from the lung tissue of infected mice that possess an intact innate immune signaling system and expressing ADAR1. But mice genetically deficient in the IKKε kinase and defective in the induction of a class of IFN induced genes including ADAR1 displayed infrequent A-to-G transitions compared to control mice (tenOever et al. 2007). Thus, sequence analyses both of measles virus, a member of the Paramyxoviridae that multiplies in the cytoplasm, and of influenza A virus, a segmented negative-strand virus that is a member of the Orthomyxoviridae that replicates and transcribes viral RNA in the nucleus (Samuel 2010a), reveal nucleotide substitutions characteristic of ADAR editing found under physiologic conditions where elevated levels of ADAR1 p150 would be expected (tenOever et al. 2007; Suspéne et al. 2008, 2011). In addition, following infection with influenza A PR8 or Udorn strains, quantitative analysis of the nucleolar proteome also showed an increase in the nucleolar ADAR1 p110 and p150 levels (Emmott et al. 2010).
Loss of function approaches have provided direct evidence consistent with the conclusion that ADAR1 plays an important role as a host restriction factor for controlling measles virus-host interactions (Toth et al. 2009; Ward et al. 2011). The effect of ADAR1 deficiency on the growth of the Moraten vaccine strain and virus-induced cell death was assessed with human HeLa cells made stably deficient in ADAR1 p110 and p150 through short hairpin RNA-mediated knockdown (Toth et al. 2009). The growth of MV mutants lacking expression of C or V accessory proteins was decreased in the ADAR1-deficient knockdown cells compared to ADAR1-sufficient cells. However, MV-induced apoptosis was enhanced in the ADAR1-deficient cells. Furthermore, the C mutant-infected ADAR1-sufficient cells when ADAR1 did not protect against apoptosis, caspase cleavage of the p150 but not p110 protein was observed. The enhanced apoptosis observed in the ADAR1 knockdown cells correlated with enhanced activation of PKR and IRF3. To further evaluate the role of the p150 isoform in the host response to MV infection, mouse embryo fibroblast cells stably expressing the SLAM receptor for MV and genetically deficient in p150 were compared to wild-type (WT) MEFs following infection with the Edmonston vaccine strain (Ward et al. 2011). Deletion of the p150 isoform of ADAR1 increased susceptibility of MEF cells to MV infection. The p150 null MEFs but not the WT MEFs displayed extensive MV-induced CPE following infection. While at early times after infection the yield of infectious progeny was reduced in the p150 null compared to WT MEFs, at the later times the yields were not statistically different. These results taken together indicate that, both in human HeLa and mouse MEF cells, ADAR1 plays an anti apoptotic role in the context of infection with MV vaccine strains (Toth et al. 2009; Ward et al. 2011).
The protection provided by the p150 isoform of ADAR1 against virus-induced cytopathic effects seen in MEF cells is not limited to measles virus of the Morbillivirus genus (Ward et al. 2011). Additional members of the Paramyxoviridae family including Newcastle disease virus (Avulavirus genus), Sendai virus (Respirovirus genus) and canine distemper virus (Morbillivirus genus) showed pronounced cytopathology in Adar1 p150−/− MEFs that was not observed in WT MEFs. The p150 isoform also protected against CPE in MEFs infected with the mouse-adapted influenza A WSN strain, a member of the Orthomyxoviridae. Reconstitution of the Adar1 p150−/− MEFs with mouse ADAR1 completely protected against development of CPE by the various myxoviruses. Taken together, these results indicate a general protective role of the p150 isoform of ADAR1 against infection with paramyxoviruses and orthomyxoviruses in MEFs (Ward et al. 2011). Three additional paramyxoviruses, human respiratory syncytial virus (RSV, Pneumovirus genus), human parainfluenza virus 3 (HPIV3, Respirovirus genus) and mumps virus (Rubulavirus genus) also have been shown to undergo mutation consistent with the A-to-I editing action of an ADAR (Murphy et al. 1991; Rueda et al. 1994; Martínez and Melero 2002; Amexis et al. 2002; Chambers et al. 2009). In the case of RSV, biased A-to-G substitutions that caused the loss of neutralization epitopes of the glycoprotein G were identified in G gene of antibody escape virus mutants, but not in the F gene glycoprotein (Rueda et al. 1994; Martínez and Melero 2002). With HPIV3, numerous A-to-G and U-to-C transitions were detected in the 3’-end of HPIV3 RNA recovered from long term persistently infected cells in culture (Murphy et al. 1991). ADAR catalyzed deamination also has been implicated in the sequence changes seen in the Jeryl Lynn vaccine strain of mumps virus (Amexis et al. 2002; Chambers et al. 2009).
Rhabdoviridae
Vesicular stomatitis virus (VSV), a member of the Rhabdoviridae family, has a negative-sense ssRNA genome of ~11-kb and multiplies in the cytoplasm by a scheme similar to measles virus (Knipe et al. 2007). However, in contrast to the enhanced virus-induced cytotoxicity effects mediated by ADAR1 deficiency seen for paramyxoviruses as illustrated by measles virus (Toth et al. 2009; Ward et al. 2011), ADAR1 deficiency did not affect the replication of VSV in the absence of IFN treatment (Li et al. 2010; Ward et al. 2011). Likewise, overexpression of either ADAR1 or ADAR2 did not significantly affect VSV growth even when the virus was passaged for ten rounds of growth in HEK 293 cells engineered to overexpress either p150 ADAR1 or ADAR2 (Li et al. 2010). The lack of a readily demonstrable effect on VSV multiplication of either ADAR1 overexpression, or ADAR1 deficiency by genetic knockout or RNAi knockdown, is somewhat surprising given the similarities between VSV and MV in genome structure and replication mechanism. ADAR deficiency leads to enhanced virus-induced cytotoxicity in the case of MV (Toth et al. 2009; Ward et al. 2011), but not VSV (Li et al. 2010; Ward et al. 2011) which by comparison is more cytopathic than is MV even in the presence of ADAR. However, two reports suggest that ADAR-mediated hypermutation may occur albeit at very low frequency of detection in mammalian or insect cells infected with rhabdoviruses. Clustered A-to-G transitions over a short A-rich sequence region have been described for a VSV defective interfering particle (O’Hara et al. 1984) and also for the Drosophilia sigma rhabdovirus PP3 gene region (Carpenter et al. 2009).
Bunyaviridae and Arenaviridae
Bunyaviridae and Arenaviridae have single-stranded RNA genomes, but they are segmented. Viruses in the family Bunyaviridae contain three ssRNA segments (S, M, L) whereas Arenaviridae members have two ssRNA segments (S, L). While most members of the Bunyaviridae are negative-stranded, phleboviruses including Rift Valley fever virus (RVFV) use an ambisense coding strategy for the S-segment that includes decoded sequence in both the negative and positive senses, as do members of the Arenaviridae family including lymphocytic choriomeningitis virus (LCMV) for the S and L segments (Knipe et al. 2007). Mutations characteristic of ADAR editing have been described in RVFV and LCMV infections (Grande-Pérez et al. 2002; Zahn et al. 2007; Suspéne et al. 2008). The 3DI-PCR strategy was used to demonstrate that infection of MRC5 cells with a RVFV strain that lacks a functional NSs protein gave rise to RVFV genomes with extensive A-to-G editing of viral RNA, but when the infection was carried out in Vero cells only quasispecies variation was seen (Suspéne et al. 2008). Vero cells are defective for IFN-α and β production, whereas MRC5 cells display a competent IFN response. RVFV with defective NSs protein was used, as wild-type NSs blocks IFN production and mediates degradation of the PKR kinase (Billecocq et al. 2004; Habjan et al. 2009).
LCMV has two ssRNA genome segments, S (~3.5-kb) and L (~7.2-kb), and among the LCMV S-segment gene products are the glycoprotein precursor that gives rise to GP1 and GP2. When the mutation rate and pattern of the GP region of genomic clones of LCMV were analyzed at early and late times after infection of L929 mouse fibroblast cells with the WE virus strain, the mutations seen did not exhibit any specific pattern at 2 days after infection. But by 7 days after infection a distinct A-to-G mutation bias emerged that gave rise to nonfunctional glycoprotein at a high frequency (Zahn et al. 2007). The preference for A-to-G (U-to-C) substitutions was also seen in spleen tissue of LCMV infected mice with a higher rate of mutation found at late time points. LCMV did not antagonize ADAR1 activity, but rather infection upregulated ADAR1 p150 in L929 cells and mice (Zahn et al. 2007). LCMV replicates in the cytoplasm, and ADAR1 p150 is localized in part in the cytoplasm. These results taken together suggest that ADAR1 is antiviral in the context of LCMV infection at late times after infection. At early times after infection, however, no significant differences were observed between wild-type and ADAR1 p150−/− mutant MEFs in the growth of the LCMV clone 13 strain of virus (Ward et al. 2011).
Hepatitis delta virus
Hepatitis delta virus (HDV) possesses a circular ~1.7-kb ssRNA negative-sense RNA genome that forms a rod-like imperfect duplex that is extensively base-paired (Taylor, 2003). HDV is a defective satellite of hepatitis B virus, and is dependent upon HBV helper function that provides the HBV envelope surface antigen as a component of the HDV particle. HDV genome replication takes place in the nucleus, and is dependent upon small delta antigen (HDAg-S). Late during replication large delta antigen (HDAg-L) is made that is required for HDV virion production. A-to-I editing of HDV RNA at the amber/W site, which converts an UAG termination codon to an UGG tryptophan (W) codon allows for the production of HDAg-L as reviewed by Casey in this volume (Casey 2011). While both ADAR1 and 2 are able to catalyze the editing of HDV RNA, ADAR1 is believed to be the enzyme primarily responsible for editing the amber/W site. Efficient editing of HDV RNA is restricted to the amber/W site despite the ~70% duplex character of HDV RNA. Under normal physiologic conditions A-to-I editing at the amber/W site is proviral.
Control of the rate and extent of A-to-I editing at the amber/W site however is essential for normal HDV replication (Casey 2011). Under conditions of ADAR1 overexpression, high levels of amber/W site editing leads to aberrant HDAg-L expression that aborts replication prematurely, and hence editing is antiviral. Elevated expression of p150 ADAR1 would be expected, for example, under conditions of IFN treatment as a therapy for HBV. IFNα induces ADAR1, increases editing and decreases HDV RNA replication (Patterson and Samuel 1995; Wong et al. 2003; Hartwig et al. 2004, 2006). However, HDV also has been described to impair IFN signaling (Pugnale et al. 2009) which would be anticipated to impair the induction of p150 ADAR1. The balance between pro- and anti-viral effects of ADAR editing of HDV RNA no doubt are determined by the combination of virus genotype, host cell and relative IFN induction and action levels achieved.
4.2 Positive-stranded RNA viruses
Positive-stranded RNA viruses include those viruses with positive-sense ssRNA genomes, that is, their genomic RNA is the polarity of mRNA and is decoded directly by translation to protein. Among the positive-stranded viruses is hepatitis C virus (HCV), a member of the Flaviviridae family. HCV possesses a ~9.6-kb positive-sense ssRNA genome enclosed within an enveloped virion that encodes a polyprotein precursor of ~3000 amino acids which is processed by viral and cellular proteases to produce 10 mature viral proteins (Knipe et al. 2007; Samuel 2010b).
HCV infection is a major global problem, with estimates of over 100 million individuals persistently infected, and an estimated ~350,000 deaths annually due to cirrhosis and heptacellular carcinoma (Perez et al. 2006). The treatment for chronic hepatitis infection is a combination therapy of pegylated type I IFN-α and ribavirin. Among the IFN inducible genes is ADAR1 p150. The site of HCV replication is the cytoplasm, and ADAR1 p150 localizes in part to the cytoplasm. Retrospective analysis of chronic hepatitis C virus-infected patients for responsiveness to treatment with IFN and ribavirin has revealed that patient genotype, in addition to the HCV virus genotype, viral load, and cirrhosis status are important factors in determining therapy responsiveness (Hwang et al. 2006; Welzel et al. 2009). And, ADAR1 is among the genes identified that associated with the responsiveness trait when DNA polymorphisms of responders and nonresponders of Taiwanese (Hwang et al. 2006) and European (Welzel et al. 2009) origins were analyzed. In addition to the IFN-inducible ADAR1, other IFN system components including the IFN receptor, JAK1 signaling kinase and IFN induced protein 44 were noted.
So far only limited mechanistic studies have been described to assess the potential role that ADAR1 plays directly on HCV replication. In Huh7 cells stably transfected with an HCV replicon, IFNα treatment was reported to inhibit replicon expression in part through the involvement of ADAR1 (Taylor et al. 2005). Adenovirus VAI RNA, identified initially as an IFN system antagonist of PKR (Kitajewski et al. 1986; Samuel 2001), also impairs ADAR1 activity in vitro (Lei et al. 1998). Inhibition of both PKR and ADAR1 by VAI RNA stimulated HCV replicon expression and decreased the amount of I-containing RNA found in replicon cells (Taylor et al. 2005). Consistent with the notion that ADAR1 was targeted by VAI RNA to inhibit HCV replicon expression, siRNA knockdown of ADAR1 was observed to stimulate replicon expression. While the HCV replicon system has provided a valuable approach to analyze HCV RNA replication (Appel et al. 2006), the availability of the JFH infectious virus and hepatoma cell culture system now makes possible mechanistic analyses of innate immune responses in HCV virus-infected cells (Lemon 2010). The infectious HCV virus cell culture system provides an approach to further assess the role of ADAR1 in the antiviral actions of IFN. Potential targets of ADAR1 relevant to the IFN response against HCV include microRNAs as well as the RNA-dependent protein kinase PKR. IFN modulation of cellular microRNAs has been reported as a component of the HCV antiviral response (Pedersen et al. 2007). HCV is known to use the abundant liver-specific miR122 to enhance replication (Skalsky and Cullen 2010; You et al. 2011), and ADAR is known to affect both the production and targeting of some cellular micro RNAs (Wulff and Nishikura 2011). Activation of the IFN-inducible PKR by HCV impairs cap-dependent translation and production of IFN response proteins (Garaigoita et al. 2009) as well as the production of IFN (Arnaud et al. 2010), and ADAR is known to function as a suppressor of PKR activation (Nie et al. 2007; Toth et al. 2009; Li et al. 2010).
4.3 Double-stranded RNA viruses
The only viruses with double-stranded RNA genomes that are known to infect mammals are some members of the Reoviridae including the orthoreoviruses. Orthoreoviruses, commonly called reoviruses as they are the founding members of the family, are naked (non-enveloped) virions that possess a segmented dsRNA genome consisting of 10 segments of fully complementary dsRNA that fall into three size classes; the total size of the 3 L, 3 M and 4 S-sized segments is ~23.5-kbp of dsRNA (Gomatos and Tamm 1963; Joklik 1981; Knipe et al. 2007). Replication of reoviruses occurs within the cytoplasm of the infected host. RNA transcription is by a virion core-associated dsRNA-dependent ssRNA polymerase that utilizes the dsRNA segments as templates for production of plus-sense transcripts that then serve as mRNA and also as templates for synthesis of progeny dsRNA during subviral particle morphogenesis. Despite over 20-kbp of naturally occurring dsRNA, relatively little is known regarding the roles, if any, that ADARs may play in reovirus replication and pathogenesis. What is clear is that reovirus multiplication occurs to high yields in many lines of cultured mammalian cells that possess both ADARs and also functional RNA interference machinery, both of which act on dsRNA. These observations suggest that the reovirus dsRNA is shielded from the host cell’s defense machinery. Indeed, no free reovirus dsRNA is normally found in infected cells; as far as is known, reovirus dsRNA is present only enclosed in viral particles (Joklik 1981; Knipe et al. 2007). The yield of infectious Dearing strain reovirus in MEF cells genetically deficient for the cytoplasmic deaminase form, ADAR1 p150, is comparable to that seen in wild-type MEFs in the absence of IFN treatment; furthermore, type I IFN treatment reduces the yield of reovirus comparably in the Adar1 p150+/+ WT and p150−/− mutant cells (Ward et al. 2011).
4.4 Double-stranded DNA viruses
Double-stranded DNA viruses include viruses of the Polyomaviridae and Herpesviridae families. Their genomes are circular dsDNA of ~5 kbp in the case of polyoma virus and linear dsDNA of ~125 to 250 kbp dependent upon the specific herpesvirus. Transcription of viral genes in both cases occurs in the nucleus by the cellular RNA polymerase II machinery. ADAR-mediated RNA editing has been described for viral RNA transcripts of mouse polyoma virus (Liu et al. 1994; Kumar and Carmichael 1997; Gu et al. 2009) and two herpesviruses, Kaposi’s sarcoma-associated herpesvirus (Gandy et al. 2007) and Epstein-Barr virus (Iizasa et al. 2010).
Polyomaviridae
Mouse polyoma virus, mPyV, is a small DNA virus in which the naked virion capsid encloses a single ~5-kbp molecule of circular dsDNA complexed with cellular histones to form of a minichromosome. In mouse cells permissive for productive infection, the early and late regions of the viral genome are expressed bidirectionally, with early and late mRNAs transcribed from opposite strands of the DNA genome. Spliced early mRNAs encode three regulatory proteins that include large T antigen that is important for DNA replication. Spliced late mRNAs are expressed efficiently only after DNA replication and encode three capsid proteins VP1, VP2 and VP3 (Benjamin 2001; Knipe et al. 2007). Evidence has been presented that early RNA gene expression is regulated by sense-antisense interactions that result in extensive A-to-I editing of the early strand RNA transcripts at late times after infection (Liu and Carmichael 1993; Liu et al. 1994; Kumar and Carmichael 1997). Formation of dsRNA occurs in the regions of sense-antisense overlap of the early and late viral transcripts that includes overlap of the polyadenylation signals (Gu et al. 2009). Extensive A-to-G (I) sequence changes are seen in mPyV early strand RNAs present in the nucleus at late times after infection (Kumar and Carmichael 1997), consistent with the hyperediting action of an ADAR. Given the nuclear localization of mPyV transcription, either ADAR1 or ADAR2 which both are nuclear enzymes could presumably be responsible for the biased hyperediting in the 3’-overlap region of mPyV RNAs. While transient knockdown of ADAR1 in NIH3T3 cells caused a defect in early-to-late switch suggesting mPyV infection is sensitive to ADAR1 protein levels (Gu et al. 2009), the availability of MEFs genetically null in Adar1, Adar1 p150 and Adar2 should permit the unequivocal identification of which ADAR edits mPyV RNA in the nucleus (Higuchi et al. 2000; Hartner et al. 2004, 2009; Wang et al. 2004; XuFeng et al. 2009; Ward et al. 2011). ADAR-catalyzed A-to-I editing of cellular mRNAs containing dsRNA structures in their 3’-UTRs, including inverted Alu repeats in the case of cellular mRNAs in human cells, may affect localization of the RNAs (Hundley and Bass 2010). However, for mPyV, it is not yet fully clear whether it is the formation of the dsRNA in the 3’-region of overlapping polyadenylation signals or the A-to-I editing per se of the dsRNA that is the critical determinant for regulation of mPyV RNA expression.
Herpesviridae
Kaposi’s sarcoma-associated herpesvirus, KSHV or human herpesvirus 8 (HHV-8), is associated with Kaposi’s tumors seen in immunosuppressed patients including, for example, AIDS patients (Knipe et al. 2007; Mesri et al. 2010). In the case of KSHV, a viral transcript is edited in a manner that affects both a protein coding sequence and a microRNA (Gandy et al. 2007). During lytic infection most KSHV viral genes are transcribed in cascades with temporal regulation, whereas during latency only a few viral genes are expressed and among the most abundant is the K12 kaposin transcript. The K12 transcript encodes three kaposin proteins (A, B, C) and a microRNA (miR-K10) and has oncogenic potential (Damania 2004). A-to-I editing occurs at genome position117990 in the K12 transcript, and in the kaposin A ORF, changes serine at position 38 to glycine. The nt substitution also changes position 2 at the 5’end of miR-K10, potentially altering targeting. Editing levels are increased nearly 10-fold following treatment with phorbol ester or sodium butyrate to activate lytic virus replication. Transcripts containing an A at 117990 are tumorigenic, while those with a G corresponding to edited RNAs with I are not tumorigenic as measured by focus formation in Rat3 cells and tumor production in nude mice. ADAR1, at least the p110 form expressed using baculovirus and purified from Sf9 insect cells, efficiently edits the K12 transcript (Gandy et al. 2007).
Epstein-Barr virus, EBV or human herpes virus 4 (HHV-4), is a lymphotropic herpesvirus that can infect and transform a range of human B cells and is associated with latent infections and diseases including infectious mononucleosis and Burkitt’s lymphoma (Knipe et al. 2007). EBV encodes more than 20 microRNAs and among them is the BART6 miRNA (Pfeffer et al. 2004; Skalsky and Cullen 2010). In the case of EBV, four viral miRNAs including BART6 miRNA primary transcripts are edited in latently EBV-infected cells (Iizasa et al. 2010). Primary miRNA transcripts are processed by the Drosha and Dicer endonucleases that act together with dsRNA binding proteins to generate the mature 20 to 22-nt miRNAs that function in silencing of gene expression (Filipowicz et al. 2008; Skalsky and Cullen 2010). The BART6 viral miRNA targets the Dicer nuclease at multiple sites in the 3’-UTR of Dicer mRNA. A-to-I editing of BART6 dramatically reduces loading of miRs onto the RISC silencing complex and inhibits silencing activity. The editing analysis of EBV primary miRNAs in EBV-infected human lymphoblastoid, Daudi Burkitt lymphoma and nasopharyngeal carcinoma cell lines suggest that EBV miR-BART6 RNAs play important roles in the regulation of viral replication and latency, and that A-to-I editing of BART6 may be an adaptive selection to counteract the targeting of Dicer by miR-BART6 (Iizasa et al. 2010).
Poxviridae and Adenoviridae
Finally, gene products of two viruses with linear dsDNA genomes, vaccinia virus of the Poxviridae that multiplies in the cytoplasm and adenovirus 5 of the Adenoviridae that multiplies in the nucleus, have been demonstrated to antagonize ADAR1 enzymatic activity. The vaccinia virus E3L protein and the adenovirus VAI RNA inhibit A-to-I editing activity of ADAR1 (Lei et al. 1998; Liu et al. 2001). However, it is not known whether vaccinia virus or adenovirus viral RNA is edited by an ADAR. Interestingly, the Z-DNA binding domain present in the N-terminal region of ADAR1 p150 was originally described as a poxvirus E3L homology domain (Patterson and Samuel, 1995). The Z-DNA binding domain present in the N-terminal region of vaccinia virus E3L plays a role in viral pathogenesis; mutations that decrease Z-DNA binding correlate with decreased viral pathogenicity in the mouse model (Kim et al. 2003). The E3L protein also binds dsRNA and mediates IFN resistance, promotes vaccinia virus growth, and impairs virus-mediated apoptosis. Loss of PKR expression in HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis and largely abolishes virus-induced apoptosis (Zhang et al. 2008). ADAR1 suppresses activation of PKR (Nie et al. 2007; Toth et al. 2009) as earlier discussed. Adenovirus VAI RNA, in addition to antagonizing PKR activation (Kitajewski et al. 1986), also antagonizes the activity of ADAR1 (Lei et al. 1998; Taylor et al. 2005).
4.2 Retroviruses
Retroviridae. Retroviruses are enveloped viruses that possess a positive-stranded RNA genome of about 7 to 10-kbp dependent upon the specific retrovirus. The single-stranded positive-sense RNA genome is converted by the process of reverse transcription into a dsDNA provirus that is subsequently integrated into the host cell’s genome. Retroviral RNAs then are produced by the host cell RNA polymerase II from the integrated provirus dsDNA template and processed by the host cell splicing, capping and polyadenylation machineries (Knipe et al. 2007). ADARs and A-to-I editing have been reported to affect retrovirus-host cell interactions, most recently in studies with human immunodeficiency virus (HIV), a member of the lentivirus genus of the Retroviridae family. ADARs are reported to generally display an HIV proviral effect (Phuphuakrat et al. 2008; Doria et al. 2009, 2011; Clerzius et al. 2009). Sequence changes consistent with the catalytic activity of an ADAR, A-to-I (G) mutations, are described not only for HIV but first were reported for two avian retroviruses (Felder et al. 1994; Hajjar and Linial 1995).
Activation of CD4+ T-lymphocytes leads to increased expression of ADAR1 but not of ADAR2 (Phuphuakrat et al. 2008). ADAR1 overexpression was shown to increase HIV production as measured by p24 Gag protein expression in a manner that required ADAR catalytic activity, whereas the silencing of ADAR1 inhibited HIV production (Phuphuakrat et al. 2008). When two regions of HIV RNA with dsRNA character were examined, the TAR and RRE cis-regulatory elements, sequences around TAR did not show any repetitive editing, whereas 8 of 30 clones showed A-to-G mutations in a 3-nt site in the RRE region (Phuphuakrat et al. 2008). An earlier study had shown that the TAR RNA stem-loop structure of HIV was an editing substrate for ADARs in a different assay, microinjected Xenopus oocytes (Sharmeen et al. 1991). Extending the observations of Phuphuakrat et al. (2008), ADAR1 expression also was shown to not only increase during HIV replication in lymphocytes, but ADAR1 was found to interact with and inhibit the RNA-dependent protein kinase PKR, and to reverse the PKR-mediated inhibition of HIV LTR expression and viral production (Clerzius et al. 2009). The proviral effect of ADAR1 in these transfection analyses, however, did not require the catalytic domain of the deaminase, but did require the Z-DNA and dsRNA nucleic acid binding domains of ADAR1, suggesting that under this experimental design, either nucleic acid sequestration or protein-protein interaction or both activities of ADAR1 were sufficient to mediate the proviral effects and that deamination of A-to-I was not necessary (Clerzius et al. 2009).
An independent study found that ADAR1 stimulated HIV replication by both A-to-I editing-dependent and editing-independent mechanisms (Doria et al. 2009). Overexpression of ADAR1 by transfection was also reported to increase HIV protein production in a manner independent of catalytic activity, but virions produced in the presence of overexpressed catalytically active ADAR1 were released more efficiently and display enhanced infectivity in challenge assays. Sequence analyses revealed editing of HIV RNAs in the 5’-UTR region shared by HIV RNAs as well as the Tat and Rev coding sequences in ADAR1-transfected cells, but in ADAR2-transfected cells only the 5’-UTR sequence changes were observed (Doria et al. 2009, 2011). Analogous to ADAR1, ADAR2 also enhances the release of progeny HIV virions by an editing-dependent mechanism, but unlike ADAR1, ADAR2 did not increase the infectivity of the HIV produced (Doria et al. 2011). The catalytic independent effect of ADAR1 on HIV1 expression seen under transfection conditions (Clerzius et al. 2009; Doria et al. 2009) may indeed relate to the impairment of PKR kinase activation and subsequent phosphorylation of eIF-2α, thereby increasing protein production (Nie et al. 2007, Wang and Samuel 2009).
The enhanced release of HIV virions by overexpression of either ADAR1 or ADAR2 is curious (Doria et al. 2009, 2011). Interestingly ADAR2 deficiency has been shown to impair exocytosis (Yang et al. 2010). Selective knockdown of ADAR2 expression markedly impaired glucose-stimulated insulin secretion in the rat INS-1 cells and primary pancreatic islets and significantly diminished KCl-stimulated protein secretion in rat adrenal pheochromocytoma PC12 cells. Catalytically active but not editing-deficient mutant ADAR2 could rescue the impairment in stimulated secretion from ADAR2 knockdown cells (Yang et al. 2010). An intriguing possibility is that the overexpression of either ADAR1 or ADAR2 enhances exocytotic processes in a manner that facilitates HIV virion assembly and release by budding (Ganser-Pornillos et al. 2008). Potentially also relevant to HIV replication responses, it has been described that inflammation elevates the level of ADAR1 but not ADAR2, and I-containing mRNA, in T-cell lymphocytes activated with TNFα (Yang et al. 2003).
The HIV-1 results taken together are most consistent with a proviral role of ADARs in the interactions of HIV-1 with the host as revealed from studies of cell culture systems including T-lymphocytes (Phuphuakrat et al. 2008; Doria et al. 2009, 2011; Clerzius et al. 2009). The proviral actions of ADARs with the lentivirus HIV-1, both catalytic-dependent and -independent, are in contrast to the antiretroviral actions of members of the APOBEC3 family of DNA mutator editing enzymes. APOBEC3G and some related cytidine deaminases function as host restriction factors and act in an antiviral manner to provide immunity against retroviruses, including HIV-1, as well as to protect the cell from endogenous retroelements (Chiu and Green 2008; Malim 2009).
5. Conclusions
Virus-host interactions are complex. In some situations, viruses take advantage of ADARs to enhance their replication. That is, the ADAR effect is proviral. This is illustrated by HDV amber/W editing to produce large delta antigen and by measles, vesicular stomatitis and HIV where ADAR appears to impair activation of antiviral processes including PKR and IRF3 responses. In other situations, the action of ADARs appears antiviral. This is illustrated by several myxoviruses with the ADAR-dependent protection against virus-induced CPE, and possibly by HCV and HDV under conditions of ADAR overexpression as would be expected for ADAR1 p150 in IFN-treated cells.
The details of the mechanisms by which the functional effects of ADARs can be both antiviral and proviral, dependent upon the virus-host combination, largely remain to be elucidated. Among the possible mechanisms are effects on the activation of innate immune responses, on macromolecular synthesis and degradation, and on adaptive immune surveillance (Table 2). No doubt the different consequences of ADARs, pro- or anti- viral, in part relate to functionally important differences between viral replication schemes. Functional genomic screens have revealed that many different host cell machineries are utilized by viruses during their replication. Spatio-temporal differences between viruses and how they interact with their hosts during replication, even for two different viruses that may have the capacity to multiply in the same kind of host cell, may lead to different functional outcomes attributed to ADAR actions. It is firmly established that ADARs can act directly, and in a catalytically-dependent manner on viral RNA, to alter function and subsequently the biological outcome of an infection. It also is becoming clear that ADARs may act indirectly to impact the infective process. For example, if a consequence of viral infection is the induction of ADAR1, then the induced ADAR p150 protein may act to alter innate immune signaling responses including activation of interferon and dsRNA-mediated cellular responses including PKR and IRF3. Such activations, of PKR and IRF3, subsequently would be expected to have broad effects on the host’s transcriptome and proteome.
Table 2.
Mechanisms by which ADARs might potentially affect the host response to viral infection
| Biological process | Molecular event | Reference* |
|---|---|---|
| Interferon signaling and action | ||
| PKR | Suppressed PKR activation | Nie et al. 2007; Toth et al. 2009; Wang et al. 2009; Li et al. 2010 |
| IRF3 | Suppressed IRF3 activation | Toth et al. 2009; Vitali and Scadden,2010 |
| IFN | Suppressed IFN induction | Hartner et al. 2009 |
|
Macromolecular synthesis and
degradation |
||
| Pre-mRNA splicing | Altered splicing | Reuter et al.1999; Raitskin et al.2001 |
| Ribosome decoding during translation | Amino acid substitution, termination | Cattaneo and Billeter 1992; Casey 2011 |
| RNA degradation | Cleavage of I-containing RNA | Scadden 2005; Scadden and O’Connell 2005 |
| RNA silencing | Altered miR processing or targeting | Iizasa et al. 2010; Wulff and Nishikura 2011 |
| Adaptive immunity | ||
| Immune surveillance | Antibody neutralization of viral antigens |
Rueda et al. 1994; Martínez and Melero 2002
Zahn et al. 2007 |
See text for additional references.
ADARs were discovered based on their catalytic activity, the ability to deaminate adenosine in duplex RNA structures that affects the subsequent stability of the targeted RNA. Subsequently examples were provided of highly selective adenosine deamination by ADARs within an open reading frame sequence, which gave rise to protein products with altered function because I is decoded as G instead of A leading to amino acid substitution as illustrated by HDV delta antigen and the glutamate and serotonin receptors. It is now clear, initially from computational approaches and then subsequently biochemical analyses, that a number of non-coding RNAs are also ADAR editing targets, including the expression and function of microRNAs. Finally, results have emerged to suggest that ADARs may function not only by deamination of adensoine in RNA regions of duplex structure, but also in a catalytically independent manner that presumably involves complex formation between ADARs and other proteins as interacting partners or the binding of nucleic acids by ADARs through their Z and dsRBD domains.
The ADAR proteins are fundamentally important determinants that mediate not only gene-selective but also general or non-selective effects in mammalian cells including virus-infected cells. In addition to different mechanisms by which ADARs may act in either a catalytically dependent or independent manner on qualitatively different targets, conceivably the quantitative extent of an ADAR-mediated action on the host may play a pivotal role in tipping the balance in either a pro- or anti- viral direction. For example, in cultured cells or intact animals, robust action of an ADAR may impair innate antiviral responses by either catalytically destabilizing activator RNAs of PKR, IRF3 or IFN responses, or by altering in potentially a catalytic-independent manner protein binding partner or nucleic acid binding interactions. In the whole animal, possibly the immune response to a viral pathogen is modulated in a manner that is antiviral when extensive A-to-I editing leads to hypermutation and impairment of viral protein production. But possibly a proviral response emerges when a low level of A-to-I editing occurs which does not disrupt threshold production of an essential viral protein such as a surface or envelope component, but instead alters the epitope structure in a manner that leads to escape from the host’s immune surveillance network. Future studies of the ADARs will likely continue to provide us with many surprises and new insights into biological processes in the context of understanding how they modulate the outcome of virus-host interactions differently for different viruses-host combinations.
Acknowledgements
This work was supported by the National Institutes of Health, Research Grants AI-12520 and AI-20611. I would like to thank Drs. Cyril George and Christian Pfaller for their helpful comments and the present and past members of the Samuel Laboratory together with the many investigators in the ADAR and innate immunity fields whose work made this chapter possible.
References
- Agranat L, Raitskin O, Sperling J, Sperling R. The editing enzyme ADAR1 and the mRNA surveillance protein hUpf1 interact in the cell nucleus. Proc Natl Acad Sci USA. 2008;105:5028–5033. doi: 10.1073/pnas.0710576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amexis G, Rubin S, Chizhikov V, Pelloquin F, Carbone K, Chumakov K. Sequence diversity of Jeryl Lynn strain of mumps virus: quantitative mutant analysis for vaccine quality control. Virology. 2002;300:171–179. doi: 10.1006/viro.2002.1499. [DOI] [PubMed] [Google Scholar]
- Appel N, Schaller T, Penin F, Bartenschlager R. From structure to function: new insights into hepatitis C virus RNA replication. J Biol Chem. 2006;281:9833–9836. doi: 10.1074/jbc.R500026200. [DOI] [PubMed] [Google Scholar]
- Arnaud N, Dabo S, Maillard P, Budkowska A, Kalliampakou KI, Mavromara P, Garcin D, Hugon J, Gatignol A, Akazawa D, Wakita T, Meurs EF. Hepatitis C virus controls interferon production through PKR activation. PLoS ONE. 2010;5:e10575. doi: 10.1371/journal.pone.0010575. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K, Lampe J, Liebert UG, Brinckmann U, ter Meulen V, Pardowitz I, Budka H, Cosby SL, Isserte S, Rima BK. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993;197:188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- Barraud P, Allain FH-T. ADAR Proteins: Double-stranded RNA and Z-DNA Binding Domains. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Benjamin TL. Polyoma virus: old findings and new challenges. Virology. 2001;289:167–173. doi: 10.1006/viro.2001.1124. [DOI] [PubMed] [Google Scholar]
- Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, Haller O. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit J, Bartlett E, Schomacker H, Surman S, Akira S, Bae YS, Collins P, Murphy B, Schmidt A. The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J Virol. 2011;85:1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, 2nd, Lowenhaupt K, Wilbert CM, Hanlon EB, Rich A. The zalpha domain of the editing enzyme dsRNA adenosine deaminase binds left-handed Z-RNA as well as Z-DNA. Proc Natl Acad Sci USA. 2000;97:13532–13536. doi: 10.1073/pnas.240464097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JA, Keegan LP, Wilfert L, O'Connell MA, Jiggins FM. Evidence for ADAR-induced hypermutation of the Drosophila sigma virus (Rhabdoviridae) BMC Genet. 2009;10:75. doi: 10.1186/1471-2156-10-75. doi:10.1186/1471-2156-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JL. Control of ADAR1 Editing of Hepatitis Delta Virus RNAs. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Billeter MA. Mutations and A/I hypermutations in measles virus persistent infections. Curr Top Microbiol Immunol. 1992;176:63–74. doi: 10.1007/978-3-642-77011-1_5. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Rebmann G, Baczko K, ter Meulen V, Bellini WJ, Rozenblatt S, Billeter MA. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986;154:97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, Schmid A, Eschle D, Baczko L, ter Meulen V, Billeter MA. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res. 2011;31:49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers P, Rima BK, Duprex WP. Molecular differences between two Jeryl Lynn mumps virus vaccine component strains, JL5 and JL2. J. Gen Virol. 2009;90:2973–2981. doi: 10.1099/vir.0.013946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YL, Greene WC. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu Rev Immunol. 2008;26:317–353. doi: 10.1146/annurev.immunol.26.021607.090350. [DOI] [PubMed] [Google Scholar]
- Clerzius G, Gélinas JF, Daher A, Bonnet M, Meurs EF, Gatignol A. ADAR1 interacts with PKR during human immunodeficiency virus infection of lymphocytes and contributes to viral replication. J Virol. 2009;83:10119–10128. doi: 10.1128/JVI.02457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C, Morgan MJ. Interferon induction and action. Annu Rev Microbiol. 1971;25:333–360. doi: 10.1146/annurev.mi.25.100171.002001. [DOI] [PubMed] [Google Scholar]
- Damania B. Oncogenic gamma-herpesviruses: comparison of viral proteins involved in tumorigenesis. Nat Rev Microbiol. 2004;2:656–668. doi: 10.1038/nrmicro958. [DOI] [PubMed] [Google Scholar]
- Desterro JM, Keegan LP, Lafarga M, Berciano MT, O'Connell M, Carmo-Fonseca M. Dynamic association of RNA-editing enzymes with the nucleolus. J Cell Sci. 2003;116:1805–1818. doi: 10.1242/jcs.00371. [DOI] [PubMed] [Google Scholar]
- Doria M, Tomaselli S, Neri F, Ciafre SA, Farace MG, Michienzi A, Gallo A. The ADAR2 Editing Enzyme Is A Novel Hiv-1 Proviral Factor. J Gen Virol. 2011 Feb 2; doi: 10.1099/vir.0.028043-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Doria M, Neri F, Gallo A, Farace MG, Michienzi A. Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection. Nucleic Acids Res. 2009;37:5848–5858. doi: 10.1093/nar/gkp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmott E, Wise H, Loucaides EM, Matthews DA, Digard P, Hiscox JA. Quantitative proteomics using SILAC coupled to LC-MS/MS reveals changes in the nucleolar proteome in influenza A virus-infected cells. J Proteome Res. 2010;9:5335–5345. doi: 10.1021/pr100593g. [DOI] [PubMed] [Google Scholar]
- Felder MP, Laugier D, Yatsula B, Dezélée P, Calothy G, Marx M. Functional and biological properties of an avian variant long terminal repeat containing multiple A to G conversions in the U3 sequence. J Virol. 1994;68:4759–4767. doi: 10.1128/jvi.68.8.4759-4767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Li H, Zhao J, Pervushin K, Lowenhaupt K, Schwartz TU, Dröge P. Alternate rRna secondary structures as regulators of translation. Nat Struct Mol Biol. 2011;18:169–176. doi: 10.1038/nsmb.1962. [DOI] [PubMed] [Google Scholar]
- Field AK, Tytell AA, Lampson GP, Hilleman MR. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci USA. 1967;58:1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Monti I, Mathews MB. Proteins binding to duplexed RNA: One motif, multiple functions. Trends Biochem Sci. 2000;25:241–246. doi: 10.1016/s0968-0004(00)01580-2. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Gandy SZ, Linnstaedt SD, Muralidhar S, Cashman KA, Rosenthal LJ, Casey JL. RNA editing of the human herpesvirus 8 kaposin transcript eliminates its transforming activity and is induced during lytic replication. J Virol. 2007;81:13544–13551. doi: 10.1128/JVI.01521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, Yeager M, Sundquist WI. The structural biology of HIV assembly. Curr Opin Struct Biol. 2008;18:203–217. doi: 10.1016/j.sbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaigorta U, Chisari FV. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe. 2009;6:513–522. doi: 10.1016/j.chom.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. 2 methylate or not 2 methylate: viral evasion of the type I interferon response. Nat Immunol. 2011;12:114–115. doi: 10.1038/ni0211-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Samuel CE. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci USA. 1999a;96:4621–4626. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Samuel CE. Characterization of the 5 '-flanking region of the human RNA-specific adenosine deaminase ADAR1 gene and identification of an interferon-inducible ADAR1 promoter. Gene. 1999b;229:203–213. doi: 10.1016/s0378-1119(99)00017-7. [DOI] [PubMed] [Google Scholar]
- George CX, Das S, Samuel CE. Organization of the mouse RNA-specific adenosine deaminase Adar1 gene 5'-region and demonstration of STAT1-independent, STAT2-dependent transcriptional activation by interferon. Virology. 2008;380:338–343. doi: 10.1016/j.virol.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Gan Z, Liu Y, Samuel CE. Adenosine deaminases acting on RNA (ADARs), RNA editing and interferon action. J Interferon Cytokine Res. 2011;31:99–117. doi: 10.1089/jir.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CX, Wagner MV, Samuel CE. Expression of interferon-inducible RNA adenosine deaminase ADAR1 during pathogen infection and mouse embryo development involves tissue-selective promoter utilization and alternative splicing. J Biol Chem. 2005;280:15020–15028. doi: 10.1074/jbc.M500476200. [DOI] [PubMed] [Google Scholar]
- Gomatos PJ, Tamm I. The secondary structure of reovirus RNA. Proc Natl Acad Sci USA. 1963;49:707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Macbeth MR, Beal PA. ADAR Proteins: Structure and Catalytic Activity. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_144. [DOI] [PubMed] [Google Scholar]
- Grande-Pérez A, Sierra S, Castro MG, Domingo E, Lowenstein PR. Molecular indetermination in the transition to error catastrophe: systematic elimination of lymphocytic choriomeningitis virus through mutagenesis does not correlate linearly with large increases in mutant spectrum complexity. Proc Natl Acad Sci USA. 2002;99:12938–12943. doi: 10.1073/pnas.182426999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Zhang Z, Decerbo JN, Carmichael GG. Gene regulation by sense-antisense overlap of polyadenylation signals. RNA. 2009;15:1154–1163. doi: 10.1261/rna.1608909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M, Pichlmair A, Elliott RM, Overby AK, Glatter T, Gstaiger M, Superti-Furga G, Unge H, Weber F. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J Virol. 2009;83:4365–4375. doi: 10.1128/JVI.02148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WW, Lamb RA, Choppin PW. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci USA. 1979;76:2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, Linial ML. Modification of retroviral RNA by double-stranded RNA adenosine deaminase. J Virol. 1995;69:5878–5882. doi: 10.1128/jvi.69.9.5878-5882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem. 2004;279:4894–4902. doi: 10.1074/jbc.M311347200. [DOI] [PubMed] [Google Scholar]
- Hartner JC, Walkley CR. Roles of ADARs in mouse development. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_150. [DOI] [PubMed] [Google Scholar]
- Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol. 2009;10:109–115. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig D, Schoeneich L, Greeve J, Schütte C, Dorn I, Kirchner H, Hennig H. Interferon-alpha stimulation of liver cells enhances hepatitis delta virus RNA editing in early infection. J Hepatol. 2004;41:667–672. doi: 10.1016/j.jhep.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Hartwig D, Schütte C, Warnecke J, Dorn I, Hennig H, Kirchner H, Schlenke P. The large form of ADAR 1 is responsible for enhanced hepatitis delta virus RNA editing in interferon-alpha-stimulated host cells. J Viral Hepat. 2006;13:150–157. doi: 10.1111/j.1365-2893.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Stefan M, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an ampa receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hundley HA, Bass BL. ADAR editing in double-stranded UTRs and other noncoding RNA sequences. Trends Biochem Sci. 2010;35:377–383. doi: 10.1016/j.tibs.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y, Chen EY, Gu ZJ, Chuang WL, Yu ML, Lai MY, Chao YC, Lee CM, Wang JH, Dai CY, Shian-Jy Bey M, Liao YT, Chen PJ, Chen DS. Genetic predisposition of responsiveness to therapy for chronic hepatitis C. Pharmacogenomics. 2006;7:697–709. doi: 10.2217/14622416.7.5.697. [DOI] [PubMed] [Google Scholar]
- Iizasa H, Wulff BE, Alla NR, Maragkakis M, Megraw M, Hatzigeorgiou A, Iwakiri D, Takada K, Wiedmer A, Showe L, Lieberman P, Nishikura K. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem. 2010;285:33358–33370. doi: 10.1074/jbc.M110.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- Joklik WK. Structure and function of the reovirus genome. Microbiol Rev. 1981;45:483–501. doi: 10.1128/mr.45.4.483-501.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawakubo K, Samuel CE. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene. 2000;258:165–172. doi: 10.1016/s0378-1119(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of c DNA for double-stranded-RNA adenosine-deaminase, a candidate enzyme for nuclear-RNA editing. Proc Natl Acad Sci USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, Jacobs BL, Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci USA. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajewski J, Schneider RJ, Safer B, Munemitsu SM, Samuel CE, Thimmappaya B, Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45:195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Knipe D, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. Fields Virology. 5th Lippincott Williams and Wilkins; Philadelphia: 2007. [Google Scholar]
- Kuhen KL, Samuel CE. Isolation of the interferon-inducible RNA-dependent protein kinase PKR promoter and identification of a novel DNA element within the 5'-flanking region of human and mouse Pkr genes. Virology. 1997;227:119–130. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Kumar M, Carmichael GG. Nuclear antisense RNA induces extensive adenosine modifications and nuclear retention of target transcripts. Proc Natl Acad Sci USA. 1997;94:3542–3547. doi: 10.1073/pnas.94.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry. 2000;39:12875–12884. doi: 10.1021/bi001383g. [DOI] [PubMed] [Google Scholar]
- Lei M, Liu Y, Samuel CE. Adenovirus VAI RNA antagonizes the RNA-editing activity of the ADAR adenosine deaminase. Virology. 1998;245:188–196. doi: 10.1006/viro.1998.9162. [DOI] [PubMed] [Google Scholar]
- Lemon SM. Induction and evasion of innate antiviral responses by hepatitis C virus. J Biol Chem. 2010;285:22741–22747. doi: 10.1074/jbc.R109.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wolff KC, Samuel CE. RNA adenosine deaminase ADAR1 deficiency leads to increased activation of protein kinase PKR and reduced vesicular stomatitis virus growth following interferon treatment. Virology. 2010;396:316–322. doi: 10.1016/j.virol.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert UG, Baczko K, Budka H, ter Meulen V. Restricted expression of measles virus proteins in brains from cases of subacute sclerosing panencephalitis. J genl Virol. 1986;67:2435–2444. doi: 10.1099/0022-1317-67-11-2435. [DOI] [PubMed] [Google Scholar]
- Liu Y, Samuel CE. Mechanism of interferon action: Functionally distinct RNA-binding and catalytic domains in the interferon-inducible, double-stranded RNA-specific adenosine deaminase. J Virol. 1996;70:1961–1968. doi: 10.1128/jvi.70.3.1961-1968.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, George CX, Patterson JB, Samuel CE. Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. J Biol Chem. 1997;272:419–4428. doi: 10.1074/jbc.272.7.4419. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wolff KC, Jacobs BL, Samuel CE. Vaccinia virus E3L interferon resistance protein inhibits the interferon-induced adenosine deaminase A-to-I editing activity. Virology. 2001;289:378–387. doi: 10.1006/viro.2001.1154. [DOI] [PubMed] [Google Scholar]
- Liu Z, Batt DB, Carmichael GG. Targeted nuclear antisense RNA mimics natural antisense-induced degradation of polyoma virus early RNA. Proc Natl Acad Sci USA. 1994;91:4258–4262. doi: 10.1073/pnas.91.10.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Carmichael GG. Polyoma virus early-late switch: regulation of late RNA accumulation by DNA replication. Proc Natl Acad Sci USA. 1993;90:8494–8498. doi: 10.1073/pnas.90.18.8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Batt DB, Carmichael GG. Targeted nuclear antisense RNA mimics natural antisense-induced degradation of polyoma virus early RNA. Proc Natl Acad Sci USA. 1994;91:4258–4262. doi: 10.1073/pnas.91.10.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Gommans WM. Novel exon of mammalian ADAR2 extends open reading frame. PLoS One. 2009;4:e4225. doi: 10.1371/journal.pone.0004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas S, Rich A, Nishikura K. A-to-I RNA Editing: Recent News and Residual Mysteries. J Biol Chem. 2003;278:1391–1394. doi: 10.1074/jbc.R200025200. [DOI] [PubMed] [Google Scholar]
- Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, Melero JA. A model for the generation of multiple A to G transitions in the human respiratory syncytial virus genome: predicted RNA secondary structures as substrates for adenosine deaminases that act on RNA. J Gen Virol. 2002 Jun;83:1445–55. doi: 10.1099/0022-1317-83-6-1445. 2002. Pt 6. [DOI] [PubMed] [Google Scholar]
- McAllister CS, Toth AM, Zhang P, Devaux P, Cattaneo R, Samuel CE. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J Virol. 2010;84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SJ, Thomis DC, Samuel CE. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology. 1992;188:47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- McCormack SJ, Samuel CE. Mechanism of interferon action: RNA-binding activity of full-length and R-domain forms of the RNA-dependent protein kinase PKR--determination of KD values for VAI and TAR RNAs. Virology. 1995;206:511–519. doi: 10.1016/s0042-6822(95)80067-0. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittaz L, Scott HS, Rossier C, Seeburg PH, Higuchi M, Antonarakis SE. Cloning of a human RNA editing deaminase (ADARB1) of glutamate receptors that maps to chromosome 21q22.3. Genomics. 1997;41:210–217. doi: 10.1006/geno.1997.4655. [DOI] [PubMed] [Google Scholar]
- Moss WJ, Griffin DE. Global measles elimination. Nat Rev Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, Dimock K, Kang CY. Numerous transitions in human parainfluenza virus 3 RNA recovered from persistently infected cells. Virology. 1991;181:760–763. doi: 10.1016/0042-6822(91)90913-v. [DOI] [PubMed] [Google Scholar]
- Nakhaei P, Genin P, Civas A, Hiscott J. Rig-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Nie Y, Hammond GL, Yang JH. Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J Virol. 2007;81:917–923. doi: 10.1128/JVI.01527-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. 2010;79:321–349. doi: 10.1146/annurev-biochem-060208-105251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–1397. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P, Nichol S, Horodyski F, Holland J. Vesicular stomatitis virus defective interfering particles can contain extensive genomic sequence rearrangements and base substitutions. Cell. 1984;36:915–924. doi: 10.1016/0092-8674(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Modeling subacute sclerosing panencephalitis in a transgenic mouse system: uncoding pathogenesis of disease and illuminating components of immune control. Curr Top Microbiol Immunol. 2009;330:31–54. doi: 10.1007/978-3-540-70617-5_2. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Lewicki H, Thomas D, Tishon A, Dales S, Patterson J, Manchester M, Homann D, Naniche D, Holz A. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- O’Neill LA. DNA makes RNA makes innate immunity. Cell. 2009;138:428–430. doi: 10.1016/j.cell.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Paro S, Li X, O’Connell MA, Keegan LP. Regulation and Functions of ADAR in Drosophila. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_152. [DOI] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: Evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Thomis DC, Hans SL, Samuel CE. Mechanism of interferon action - double-stranded RNA-specific adenosine-deaminase from human-cells is inducible by alpha-interferon and gamma-interferon. Virology. 1995;210:508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- Patterson JB, Cornu TI, Redwine J, Dales S, Lewicki H, Holz A, Thomas D, Billeter MA, Oldstone MB. Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease. Virology. 2001;291:215–225. doi: 10.1006/viro.2001.1182. [DOI] [PubMed] [Google Scholar]
- Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–822. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Phuphuakrat A, Kraiwong R, Boonarkart C, Lauhakirti D, Lee TH, Auewarakul P. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins. J Virol. 2008;82:10864–10872. doi: 10.1128/JVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res. 2011;31:59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- Placido D, Brown BA, Lowenhaupt K, Rich A, Athanasiadis A. A left-handed RNA double helix bound by the Z alpha domain of the RNA-editing enzyme ADAR1. Structure. 2007;15:395–404. doi: 10.1016/j.str.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. Crm1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol Cell Biol. 2001;21:7862–7871. doi: 10.1128/MCB.21.22.7862-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugnale P, Pazienza V, Guilloux K, Negro F. Hepatitis delta virus inhibits alpha interferon signaling. Hepatol. 2009;49:398–406. doi: 10.1002/hep.22654. [DOI] [PubMed] [Google Scholar]
- Raitskin O, Cho DS, Sperling J, Nishikura K, Sperline R. RNA editing activity is associated with spliceing factors in InRNP particles: The nuclear pre-mRNA processing machinery. Proc Natl Acad Sci USA. 2001;98:6571–6576. doi: 10.1073/pnas.111153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Rebagliati MR, Melton DA. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987;48:599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rueda P, García-Barreno B, Melero JA. Loss of conserved cysteine residues in the attachment (G) glycoprotein of two human respiratory syncytial virus escape mutants that contain multiple A-G substitutions (hypermutations) Virology. 1994;198:653–662. doi: 10.1006/viro.1994.1077. [DOI] [PubMed] [Google Scholar]
- Rueter SM, Dawson RT, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 2007;316:253–292. doi: 10.1007/978-3-540-71329-6_13. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Mechanism of interferon action: Phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979;76:600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Innate immunity minireview series: making biochemical sense of nucleic acid sensors that trigger antiviral innate immunity. J Biol Chem. 2007;282:15313–15314. doi: 10.1074/jbc.R700013200. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Thematic minireview series: toward a structural basis for understanding influenze virus-host cell interactions. J Biol Chem. 2010a;285:28399–28401. doi: 10.1074/jbc.R110.149625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Thematic minireview series: elucidating hepatitis C virus-host interactions at the biochemical level. J Biol Chem. 2010b;285:22723–22724. doi: 10.1074/jbc.R110.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology. 2011;411:180–193. doi: 10.1016/j.virol.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Wells KS, Emeson RB. Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proc Natl Acad Sci USA. 2003;100:14018–14023. doi: 10.1073/pnas.2336131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis PT, Ying S, Xu R, Yu XF. STAT1-independent call type-specific regulation of antiviral APOBEC3G by IFN-alpha. J Immunol. 2006;177:4530–4540. doi: 10.4049/jimmunol.177.7.4530. [DOI] [PubMed] [Google Scholar]
- Scadden AD. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat Struct Mol Biol. 2005;12:489–496. doi: 10.1038/nsmb936. [DOI] [PubMed] [Google Scholar]
- Scadden AD, O'Connell MA. Cleavage of dsRNAs hyper-edited by ADARs occurs at preferred editing sites. Nucleic Acids Res. 2005;33:5954–5964. doi: 10.1093/nar/gki909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schmid A, Spielhofer P, Cattaneo R, Baczko K, ter Meulen V, Billeter MA. Subacute sclerosing panencephalitis is typically characterized by alterations in the fusion protein cytoplasmic domain of the persisting measles virus. Virology. 1992;188:910–915. doi: 10.1016/0042-6822(92)90552-z. [DOI] [PubMed] [Google Scholar]
- Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Z domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol. 2011 Feb 9; doi: 10.1128/JVI.02634-10. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MJ, Smolter PE, Westhof E. Pronounced instability of tandem IU base pairs in RNA. Nucleic Acids Res. 2004;32:1824–1828. doi: 10.1093/nar/gkh501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L, Bass B, Sonenberg N, Weintraub H, Groudine M. Tat-dependent adenosine-to-inosine modification of wild-type transactivation response RNA. Proc Natl Acad Sci USA. 1991;88:8096–8100. doi: 10.1073/pnas.88.18.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtrichman R, Heithoff DM, Mahan MJ, Samuel CE. Tissue selectivity of interferon-stimulated gene expression in mice infected with dam(+) versus dam(−) Salmonella enterica Serovar typhimurium strains. Infect Immun. 2002;70:5579–5588. doi: 10.1128/IAI.70.10.5579-5588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov D, Gardiner K. Phylogenetic comparison of the pre-mRNA adenosine deaminase ADAR2 genes and transcripts: Conservation and diversity in editing site sequence and alternative splicing patterns. Gene. 2002;299:83–94. doi: 10.1016/s0378-1119(02)01016-8. [DOI] [PubMed] [Google Scholar]
- Stewart WE. The interferon system. Springer-Verlag. 1979 [Google Scholar]
- Strehblow A, Hallegger M, Jantsch MF. Nucleocytoplasmic distribution of human RNA-editing enzyme ADAR1 is modulated by double-stranded RNA-binding domains, a leucine-rich export signal, and a putative dimerization domain. Mol Biol Cell. 2002;13:3822–3835. doi: 10.1091/mbc.E02-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel SA, Cech TR, Usman N, Beigelman L. The 2,6-diaminopurine riboside.5-methylisocytidine wobble base pair: an isoenergetic substitution for the study of G.U pairs in RNA. Biochemistry. 1994;33:13824–13835. doi: 10.1021/bi00250a037. [DOI] [PubMed] [Google Scholar]
- Suspène R, Renard M, Henry M, Guétard D, Puyraimond-Zemmour D, Billecocq A, Bouloy M, Tangy F, Vartanian JP, Wain-Hobson S. Inversing the natural hydrogen bonding rule to selectively amplify GC-rich ADAR-edited RNAs. Nucleic Acids Res. 2008;36(12):e72. doi: 10.1093/nar/gkn295. doi: 10.1093/nar/gkn295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspène R, Petit V, Puyraimond-Zemmour D, Aynaud MM, Henry M, Guétard D, Rusniok C, Wain-Hobson S, Vartanian JP. Double-stranded RNA adenosine deaminase ADAR-1-induced hypermutated genomes among inactivated seasonal influenza and live attenuated measles virus vaccines. J Virol. 2011;85:2458–2462. doi: 10.1128/JVI.02138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Samuel CE. Mechanism of interferon action: structure of the mouse PKR gene encoding the interferon-inducible RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7995–7999. doi: 10.1073/pnas.91.17.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Puig M, Darnell MER, Mihalik K, Feinstone SM. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J Virol. 2005;79:6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM. Replication of human hepatitis delta virus: Recent developments. Trends Microbiol. 2003;11:185–190. doi: 10.1016/s0966-842x(03)00045-3. [DOI] [PubMed] [Google Scholar]
- tenOever BR, Ng SL, Chua MA, McWhirter SM, García-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- Toth AM, Li Z, Cattaneo R, Samuel CE. RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR. J Biol Chem. 2009;284:29350–29356. doi: 10.1074/jbc.M109.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AM, Zhang P, Das S, George CX, Samuel CE. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog Nucleic Acid Res Mol Biol. 2006;81:369–434. doi: 10.1016/S0079-6603(06)81010-X. [DOI] [PubMed] [Google Scholar]
- Tytell AA, Lampson GP, Field AK, Hilleman MR. Inducers of interferon and host resistance. 3. Double-stranded RNA from reovirus type 3 virions (reo 3-RNA) Proc Natl Acad Sci USA. 1967;58:1719–1722. doi: 10.1073/pnas.58.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- Valentine R, Smith GL. Inhibition of the RNA polymerase III-mediated dsDNA-sensing pathway of innate immunity by vaccinia virus protein E3. J Gen Virol. 2010;91:2221–2229. doi: 10.1099/vir.0.021998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali P, Scadden AD. Double-stranded RNAs containing multiple IU pairs are sufficient to suppress interferon induction and apoptosis. Nat Struct Mol Biol. 2010;17:1043–1050. doi: 10.1038/nsmb.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RW, Smith JE, Cooperman BS, Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci USA. 1989;86:2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of Adar1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Samuel CE. Adenosine deaminase ADAR1 increases gene expression at the translational level by decreasing protein kinase PKR-dependent eIF-2alpha phosphorylation. J Mol Biol. 2009;393:777–787. doi: 10.1016/j.jmb.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zeng Y, Murray JM, Nishikura K. Genomic organization and chromosomal location of the human dsRNA adenosine deaminase gene: The enzyme for glutamate-activated ion channel RNA editing. J Mol Biol. 1995;254:184–195. doi: 10.1006/jmbi.1995.0610. [DOI] [PubMed] [Google Scholar]
- Ward SV, George CX, Welch JJ, Liou LY, Hahm B, Lewicki H, de la Torre JC, Samuel CE, Oldstone MB. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc Natl Acad Sci USA. 2011;108:331–336. doi: 10.1073/pnas.1017241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weier HUG, George CX, Greulich KM, Samuel CE. The interferon-inducible, double-stranded RNA-specific adenosine deaminase gene (DSRAD) maps to human chromosome 1q21.1-21.2. Genomics. 1995;30:372–375. doi: 10.1006/geno.1995.0034. [DOI] [PubMed] [Google Scholar]
- Weier HUG, George CX, Lersch RA, Breitweser S, Cheng JF, Samuel CE. Assignment of the RNA-specific adenosine deaminase gene (Adar) to mouse chromosome 3f2 by in situ hybridization. Cytogenet. Cell Genet. 2000;89:214–215. doi: 10.1159/000015615. [DOI] [PubMed] [Google Scholar]
- Welzel TM, Morgan TR, Bonkovsky HL, Naishadham D, Pfeiffer RM, Wright EC, Hutchinson AA, Crenshaw AT, Bashirova A, Carrington M, Dotrang M, Sterling RK, Lindsay KL, Fontana RJ, Lee WM, Di Bisceglie AM, Ghany MG, Gretch DR, Chanock SJ, Chung RT, O'Brien TR, HALT-C Trial Group Variants in interferon-alpha pathway genes and response to pegylated interferon-Alpha2a plus ribavirin for treatment of chronic hepatitis C virus infection in the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2009;49(6):1847–1858. doi: 10.1002/hep.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SK, Sato S, Lazinski DW. Elevated activity of the large form of ADAR1 in vivo: very efficient RNA editing occurs in the cytoplasm. RNA. 2003;9:586–598. doi: 10.1261/rna.5160403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TC, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991;65:2191–2199. doi: 10.1128/jvi.65.5.2191-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TC, Ayata M, Hirano A, Yoshikawa Y, Tsuruoka H, Yamanouchi K. Generalized and localized biased hypermutation affecting the matrix gene of a measles virus strain that causes subacute sclerosing panencephalitis. J Virol. 1994;63:5464–5468. doi: 10.1128/jvi.63.12.5464-5468.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff B-E, Nishikura K. Modulation of Micro RNA Expression and Function by ADARs. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_151. [DOI] [PubMed] [Google Scholar]
- XuFeng R, Boyer MJ, Shen H, Li Y, Yu H, Gao Y, Yang Q, Wang Q, Cheng T. ADAR1 is required for hematopoietic progenitor cell survival via RNA editing. Proc Natl Acad Sci USA. 2009;106:17763–17768. doi: 10.1073/pnas.0903324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Nie Y, Zhao Q, Su Y, Pypaert M, Su H, Rabinovici R. Intracellular localization of differentially regulated RNA-specific adenosine deaminase isoforms in inflammation. J Biol Chem. 2003;278:45833–45842. doi: 10.1074/jbc.M308612200. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhao L, Gan Z, He Z, Xu J, Gao X, Wang X, Han W, Chen L, Xu T, Li W, Liu Y. Deficiency in RNA editing enzyme ADAR2 impairs regulated exocytosis. FASEB J. 2010;24:3720–3732. doi: 10.1096/fj.09-152363. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Recognition of viral nucleic acids in innate immunity. Rev Med Virol. 2010;20:4–22. doi: 10.1002/rmv.633. [DOI] [PubMed] [Google Scholar]
- You S, Murray CL, Luna JM, Rice CM. End game: Getting the most out of microRNAs. Proc Natl Acad Sci USA. 2011;108:3101–3102. doi: 10.1073/pnas.1019613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn RC, Schelp I, Utermöhlen O, von Laer D. A-to-G hypermutation in the genome of lymphocytic choriomeningitis virus. J Virol. 2007;81:457–464. doi: 10.1128/JVI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Jacobs BL, Samuel CE. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J Virol. 2008;82:840–848. doi: 10.1128/JVI.01891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]