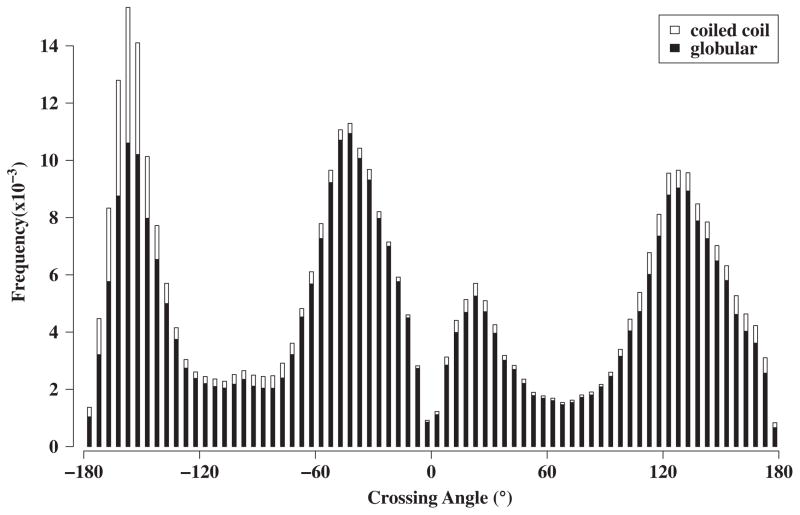
Figure 3. Crossing Angles Dependency of RPCs cliques.
Instantaneous crossing angles between two α-helices for each RPC was computed using HELANAL121 (see Materials and Methods), and the frequency distribution of helix RPCs is shown against the crossing angle. The black is from α-helices in globular proteins and the white are from coiled-coils. It is interesting to note that the distributions would be about equal if the coiled-coils were removed. Each peak corresponds to a canonical packing pattern depicted in Figure 4. The well-known peaks of coiled coils are found for anti-parallel at −165° and 25°. The other peaks occur at −30° and 150°, which also includes the shoulder at 175°.