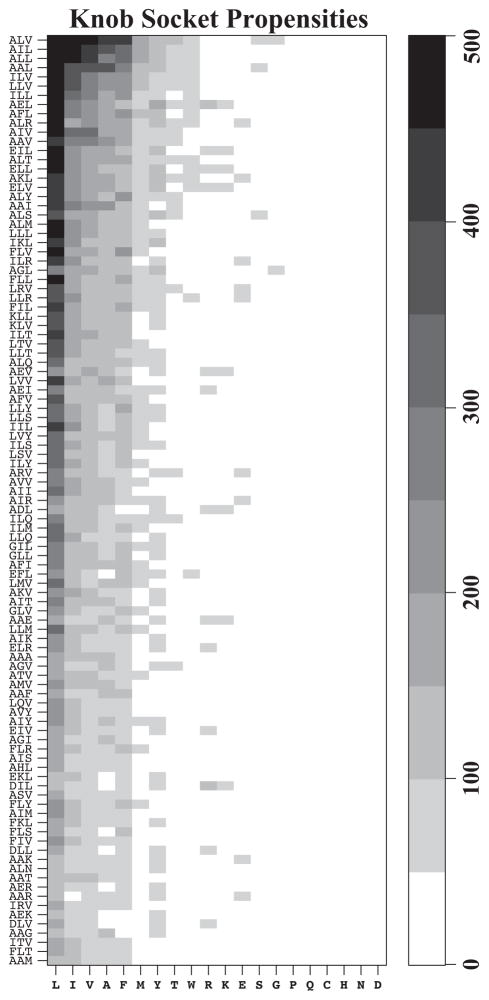
Figure 7. Knob propensities for most preferred sockets.
The heat map shows the propensities for 20 amino acid knobs B that pack with 100 most preferred XYH sockets on helical interface. The groups of three residues that make the sockets are arranged on the Y-axis from top to bottom with decreasing frequencies. Knob residues are displayed on X-axis with sequence order from left to right with high propensity knobs on left most side of the plot. Grey scaled color ramp shows the frequencies of knob residues from light (least preferred) to dark (most preferred). Non-polar beta branched residues; Leu, Ile and Val as well as small non-polar side chain Ala are most favored knobs in helix packing motifs. Amongst the bigger hydrophobic side chains, Phe is preferred over Tyr and Trp in most helix-helix interaction interfaces. Not surprisingly, most of the polar and charged residues occur with very low frequencies.