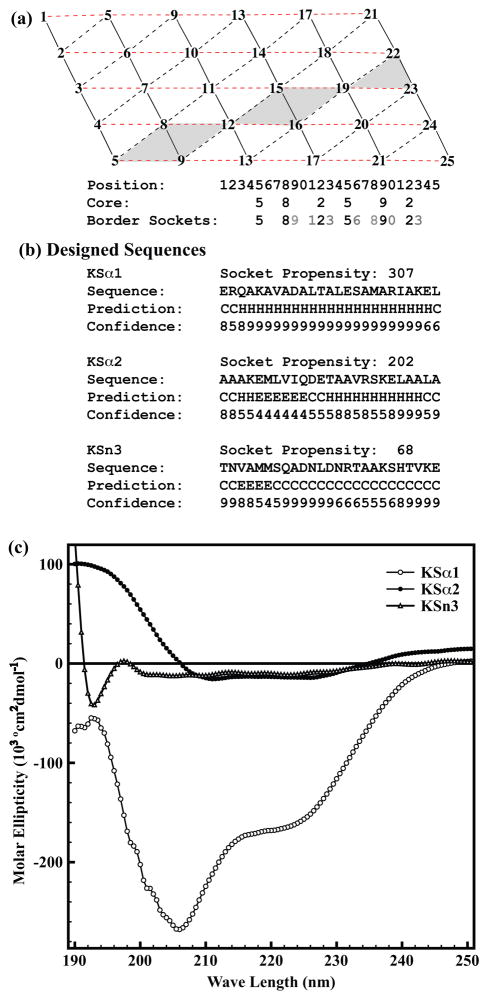
Figure 8. Knob-Socket Procedure for.
α-helix Design.
(a) The basic principle is driven by arrangement of packing on the modified α-helix lattice. First, the core residues along the path of alternating i+3 and i+4 residue position are selected (i.e. 5-8-12-15-19-22). Next, fill the positions with the amino acids to form the border sockets with desired socket propensities. For example, fill the position 9 with a residue that forms socket with residues at 5 and 8 and at 8 and 12. Repeat this for the position 16 and 23, then expand socket region gradually by choosing residues for the positions 11, 13, 18, and 20. Repeat the same procedure until all the lattice points are filled. The sequence is checked to insure sockets are created with desired socket propensities. (b) The three 25 residue sequences designed using above mentioned design strategy followed by their consensus secondary structure predictions110–115 and confidence level of predictions. For each designed sequence, helicity is shown by the calculated socket propensities. (c) Overlay of the far-UV circular dichroism (CD) spectra for the three synthesized peptides on normalized molar ellipticity scale. Two minima at 215nm and 225nm in CD spectrum are indicative of strong helicity of KSα1, which was predicted to be strongly helical with high socket propensity of 307. Although KSα2 is same as KSα1 in amino acid composition it shows very low helicity due to rearrangement of the residues in socket motif. Spectral pattern for KSn3 suggests a completely random coil conformation of the peptide that was designed with low socket propensity residues.