Figure 8.
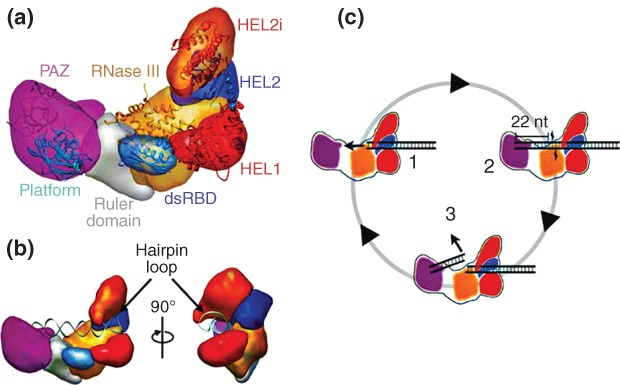
Functional interplay of human(h) Dicer domains in dsRNA processing.87 (a) Domain structure of hDicer as determined by domain-specific tagging and single molecule electron microscopy (EM). Shown are the PAZ (purple), platform (blue), ruler (gray), dsRBD (blue), ribonuclease III (RNase III) (a+b) (orange) and helicase domains, with the latter domain displayed as three subdomains HEL1 (red), HEL2 (blue), and HEL2i (orange). (b) Two views of hDicer bound to a pre-miRNA hairpin, showing the engagement of the loop by the helicase domain, and the opposing 3′-overhang end by the PAZ domain. (c) Proposed processing cycle for hDicer cleavage of dsRNA. In complex 1, the dsRNA is engaged by the helicase domain. In complex 2, cooperation of the PAZ and RNase III domains provide a 22 bp product, while the remainder of the dsRNA remains engaged with the helicase domain. In complex 3, release of the 22 bp product from the PAZ and RNase III domains allows subsequent engagement of the contiguous dsRNA segment by the RNase III and PAZ domains. This cycle explains the processivity of hDicer action, supported by the helicase domain. (Reprinted with permission from Ref 87. Copyright 2012 Macmillan Publishers Ltd)
