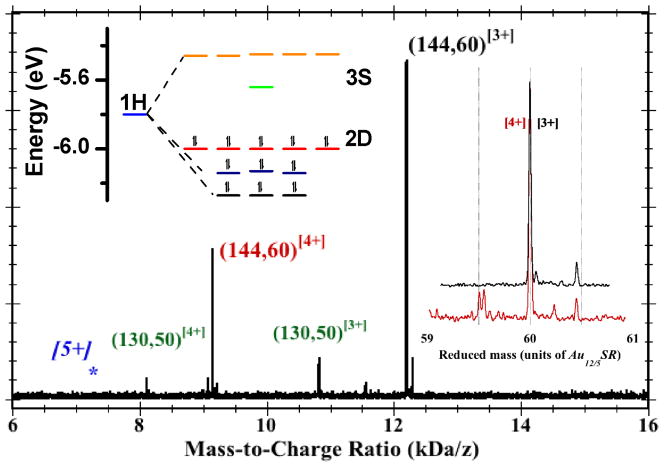
Figure 2.
Mass spectrum of a sample of purified Au144(peth)60 clusters, obtained by the electrospray-ionization (ESI) time-of-flight (ToF) method. [See SI Section.] The labelled peaks indicate the assignments to the [3+] (black) and [4+] (red) charge-states. The blue [5+] and asterisk indicate the expected location of the missing [5+] peak near 7.2 kDa/e, and the green-labeled peaks designate a residual impurity (or byproduct) also known from earlier research. Right Inset: Expanded regions of the [4+] and [3+] peaks, plotted versus the mass (rather than mass-to-charge-ratio), in units of the 60-fold unit (~ 0.610 kDa) of Au12/5(peth)1, so that the principal peaks are overlaid at reduced-mass 60. The position and width (FWHM ~ 12 Da) of these peaks are consistent with the natural isotope abundance of 13C. Minor satellite peaks toward higher masses are assigned to Cs+-adduct formation (each Cs-atom is 0.133-kDa, or 0.218 on the reduced-mass scale). The main satellite peak toward lower mass is attributed to organic disulfide (RS-SR) loss (0.274-kDa, or 0.448). Left Inset: Energy-level diagram for the frontier orbitals of Au144Cl60[4+], (HOMOs 2D10, LUMO S0) but generic to the I-Au144(SR)60[z+] systems. Seventeen (17) orbitals are classified according to united-atom (or ‘superatom’)27 representations {1H, 2D, 3S}, where {S,P,D,F,G,H, …} denote respective total angular momenta L = {0,1,2,3,4,5, …} with (2L+1)-fold orbital degeneracy. The electronic 1H-shell splits under an icosahedral field into groups of (3,3,5) each, cf. Ih-C60 or -C80 clusters. Electronic closed-shell configurations are expected for charge-states [4+] (depicted), [2+], and [8−]. The neutral state is open shell and subject to Jahn-Teller instability (deformation); see SI for details.