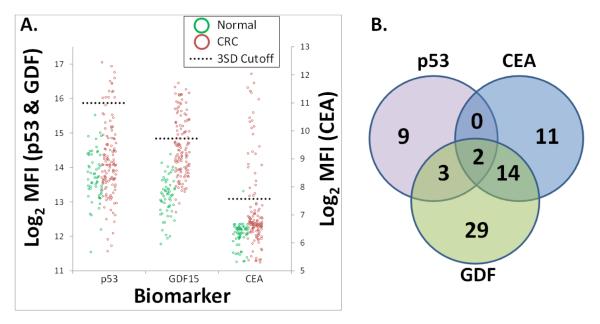
Figure 5. Diagnostic Performance of a Three Marker CRC Assay Combining Autoantibody (TAA) and Circulating Protein Detection.
(A.) 3-plex recombinant TAA and soluble protein assay on VeraCode™ beads was performed 186 patient samples (59 normal and 127 CRC). Protein TAAs were bound directly to the VeraCode™ carboxyl beads and used to assay patient serum/plasma for the presence of autoantibodies, while capture antibodies were bound to VeraCode™ carboxyl beads to assay for circulating protein markers. Individual markers are denoted on the x-axis. Individual patient samples are denoted by green circles (healthy individuals) and red circles (colorectal cancer patients). MFI= Mean Fluorescence Intensity of the BeadXpress™ instrument readout (log2 data shown). The black dotted lines indicate the diagnostic scoring cutoffs, set at 3 standard deviations above the mean of the normal patients. CEA, GDF15 and the p53 TAA were 21%, 38% and 11% sensitive and 98%, 100% and 100% specific, respectively. Composite sensitivity and specificity of all 3 biomarkers in the multiplexed assay was 54% and 98%, respectively. (B.) Venn diagram demonstrating the number of “hits”, or CRC patients which tested positive, specific to each biomarker. The overlap of circles indicates patients that were detected by more than one biomarker.