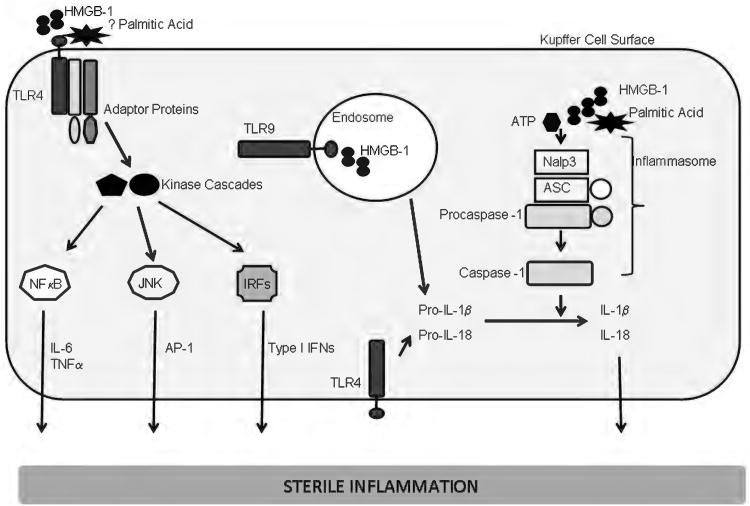
Figure 3.
Sterile inflammation in liver diseases. A model is presented for sterile inflammation in liver diseases. Palmitic acid, a toxic free fatty acid, which can activate the NLRP3 inflammasome and high mobility group box 1 (HMGB-1), a nuclear protein released from dead cells, are shown as activating damage associated molecular patterns (DAMPs). Activation of cel surface toll like receptors (TLRs)-1, 2, 4, 6, 5, or endosomal TLRs (7, 9) leads to recruitment of adaptor proteins, activation of kinase cascades that result in activation of nuclear factor κ B, c-jun N-terminal kinase, and interferon (IFN) regulatory factors. These result in transcriptional activation of several proinflammatory mediators including interleukin (IL)-6, TNF-α and Type IFNs. The inflammasome can also be activated by many endogenous DAMPs. The mechanism for this activation is not fully elucidated. Shown here is the NLRP3 (nucleotide oligomerization domain [NOD]-like receptor, pyrin domain containing 3) inflammasome. The NLRP3 gene product is the intracellular protein, Nalp3 (NACHT, LRR, and pyrin domain-containing 3). Nalp3, upon activation, recruits ASC (apoptosis-associated speck-like protein containing a CARD, also known as PYCARD), and procaspase-1, leading to the activation of caspase-1. Caspase-1 cleaves and activates the precursor forms on IL-1 β and L-18; both are subsequently secreted, and activate their receptors on target cells, resulting in the activation of proinflammatory pathways.