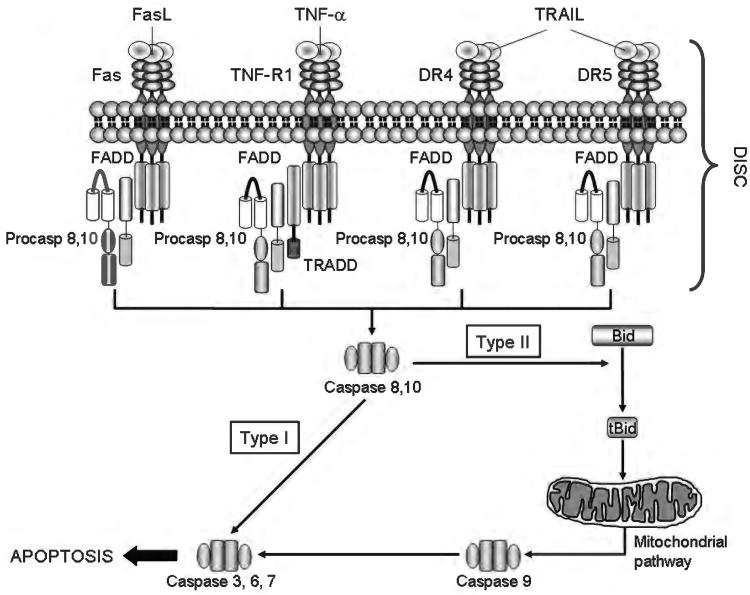
Figure 4.
The death receptors and the extrinsic pathway of apoptosis. Binding of a death ligand to its cognate receptor results in the recruitment of adaptor proteins, such as Fas-associated protein with death domain and Tumor necrosis factor receptor 1-associated death domain protein (TRADD), and procaspases 8 and/or 10, to form a multiprotein receptor complex named death inducing signaling complex (DISC). This complex provides a platform for caspase 8 and 10 to undergo autoactivation. In type I cells, active caspase 8/10 directly activate caspase 3, an effector caspase, whereas in type II cells, caspase 8 (and, perhaps, caspase 10) cleaves the BH3-only protein Bid to generate truncated Bid (tBid). tBid, in turn, cooperates with Bax or Bak to induce mitochondrial outer membrane permeabilization and to initiate the mitochondrial pathway of apoptosis (see Figure 5 for details).