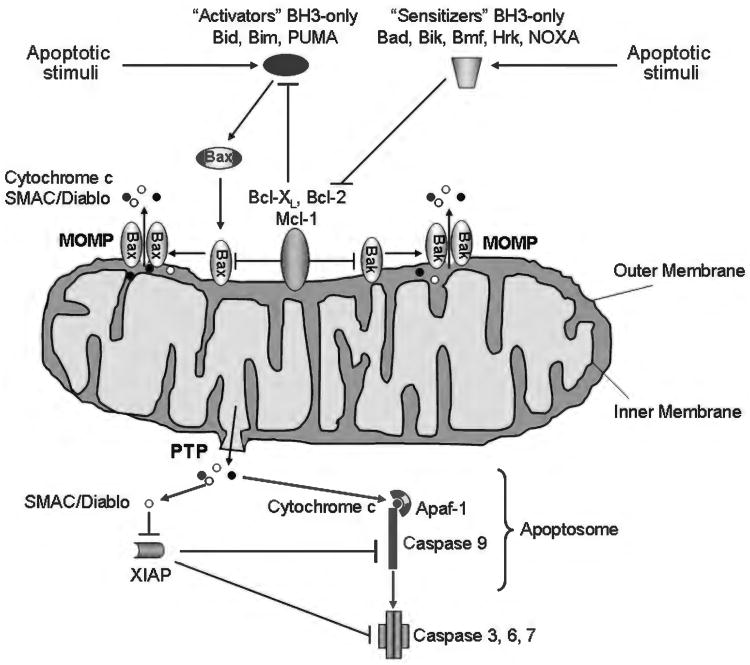
Figure 5.
The intrinsic pathway of apoptosis. Various stimuli, including UV and gamma-irradiation, endoplasmic reticulum (ER) stress, growth factor deprivation, and oxidative stress trigger the intrinsic pathway of apoptosis. This pathway requires the oligomerization of the proapoptotic members of the Bcl-2 family of protein Bax and/or Bak on the outer mitochondrial membrane, resulting in mitochondrial outer membrane permeabilization (MOMP) and release of apoptogenic factors. The oligomerization of Bax and Bak can be directly stimulated by the BH3-only proteins Bid, Bim, or PUMA (activators). Bax and Bak, as well as Bid, Bim and PUMA, are bound to and inhibited by the prosurvival Bcl-2 proteins, Bcl-2, Bcl-xL, and Mcl-1. The prosurvival function of these proteins can be repressed by the BH3-only proteins Bad, Bik, Hrk, Bmf, and NOXA (sensitizers), which displace Bid, Bim, and PUMA by binding to the prosurvival proteins. Release of Bid, Bim, and PUMA then allows activation of Bax and/or Bak. MOMP can also be achieved by the so-called mitochondrial permeability transition (MPT) initiated at the inner mitochondrial membrane through the opening of a multiprotein complex known as permeability transition pore (PTP). Several apoptogenic factors, including cytochrome c and SMAC/DIABLO, are released from the mitochondrial intermembrane space into the cytosol as a consequence of MOMP. Cytochrome c binds to the adaptor Apaf-1, and recruits procaspase-9 in a complex named apoptosome, which promotes the activation of caspase-9. Caspase-9, in turn, activates the effector caspases (caspase-3, 6, and 7). SMAC/DIABLO contributes to caspase activation by binding and inactivating the endogenous inhibitor of caspases X-chromosome linked inhibitor of apoptosis protein.