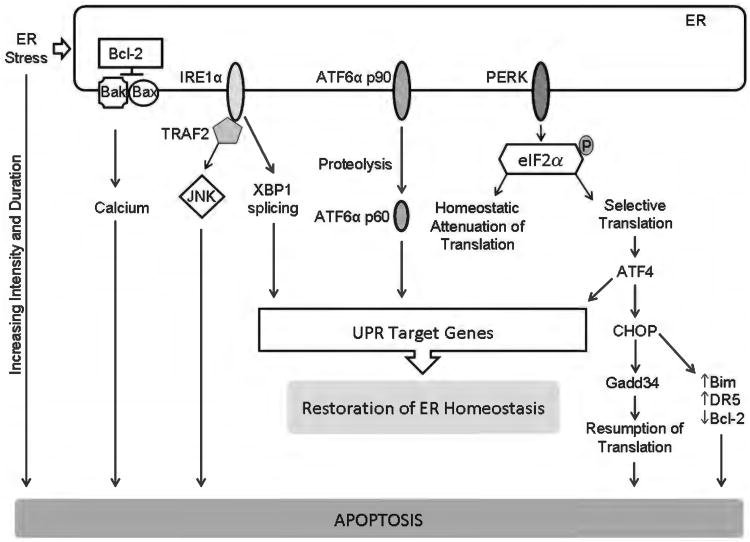
Figure 8.
Endoplasmic reticulum (ER) stress and apoptosis. ER stress activates, in parallel, three distinct ER-to-nucleus signaling pathways that are aimed at attenuating ER stress via activation of unfolded protein response (UPR) target genes with restoration of ER homeostasis. However, ER stress of increasing duration and intensity results in failure of restoration of homeostasis and apoptosis. The three canonical UPR mediators are ER transmembrane proteins; they are inositol-requiring protein 1α (IRE1α), activating transcription factor (ATF) 6α, and protein kinase RNA-like ER kinase (PERK). Active PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α) leading to an attenuation of translation. This reduces the load of nascent proteins entering the ER. Selective translation of activating ATF4 also occurs. ATF4 promotes adaptation, but also transcriptionally activates C/EBP homologous protein (CHOP). CHOP promotes ER stress-induced apoptosis via several pathways, including increasing the proapoptotic proteins Bim and death receptor 5 (DR5) and decreasing the antiapoptotic protein Bcl-2. IRE1α splices X box-binding protein 1 (XBP-1) mRNA to generate a transcription factor which leads to ER adaptation by activating a large number of UPR genes. The stress kinase, c-jun N-terminal kinase (JNK) is activated by IRE1α via the adaptor protein TNF receptor-associated factor 2 (TRAF2). Activating transcription factor 6α (ATF6α) is proteolytically cleaved in the Golgi to generate a transcription factor which also drives expression of UPR target genes. Failure of restoration of ER homeostasis due to increasing intensity and duration of ER stress results in apoptosis. Some of the recognized mediators of ER stress-induced apoptosis are the transcription factor CHOP which can repress Bcl-2 expression, and increase expression of the proapoptotic proteins Bim and DR5. ER stress-induced apoptosis can also me mediated by ER calcium release, which can be regulated by Bax, Bak, and Bcl-2. The stress kinase JNK can also activate the intrinsic apoptosis machinery.