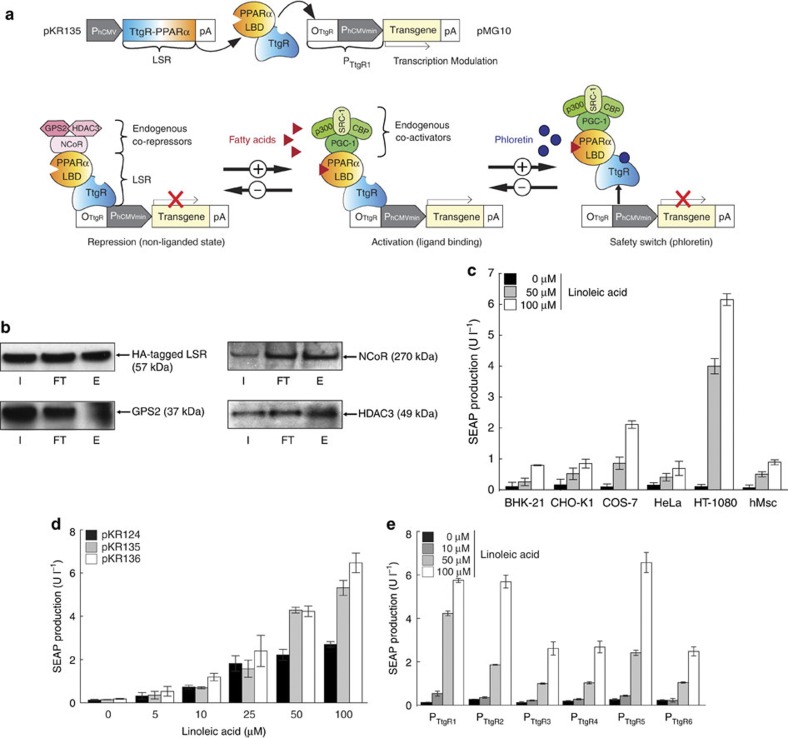
Figure 1. A synthetic fatty acid-responsive mammalian gene switch.
(a) The synthetic lipid-controlled mammalian transcription device consists of an intracellular LSR, a fusion protein combining the phloretin-responsive repressor (TtgR) and the human PPARα, which binds a TtgR-specific operator (OTtgR) linked to a minimal promoter (PhCMVmin) (PTtgR1) to control transgene expression. In the absence of fatty acids, LSR associates with an inhibitory complex (GPS2, G-protein pathway suppressor 2; HDAC, histone deacetylase; NCoR, nuclear receptor corepressor) to repress transgene expression, which switches to full induction in the presence of fatty acids when LSR associates with the activation complex (SRC1, steroid receptor coactivator 1; p300, E1A binding protein; CBP, CREB-binding protein; PGC-1, peroxisome proliferator-activated receptor-γ coactivator). Transgene expression can also be shut down by disrupting LSR-promoter binding by addition of the clinically licensed skin-penetrating apple metabolite phloretin. (b) Co-immunoprecipitation. HT-1080 were cotransfected with pKR151 (encoding HA-tagged LSR) and pMG10, and the co-repressors (NCoR, GPS2 and HDAC3) interacting with LSR were identified by protein complex CoIP after 48 h. E, eluate; FT, flow through; I, input. (c) Linoleic acid-induced SEAP expression in different cell lines. Cells were co-transfected with the LSR-encoding expression vector (pKR135; PhCMV-LSR-pA) and the PTtgR1-driven SEAP expression plasmid (pMG10; PhCMV*-1-SEAP-pA), grown in the presence of different linoleic acid concentrations, and SEAP levels were profiled in the culture supernatant after 48 h. (d,e) Optimization of the LSR’s fatty acid sensitivity. (d) HT-1080 were (co-)transfected with pMG10 (PTtgR1-SEAP-pA) and an expression vector encoding LSR under control of different constitutive promoters (pKR124, PSV40-LSR-pA; pKR135, PhCMV-LSR-pA; pKR136, PhEF1α-LSR-pA) and cultivated in the presence of increasing concentrations of linoleic acid for 48 h before SEAP was profiled in the culture supernatant. (e) HT-1080 were (co-)transfected with the LSR expression vector pKR135 (PhCMV-LSR-pA) and reporter constructs encoding SEAP under control of different LSR-specific promoter variants containing 0 (PTtgR1; pMG10 (ref. 30)), 2 (PTtgR2; pMG20 (ref. 30)), 4 (PTtgR3; pMG21 (ref. 30)), 6 (PTtgR4; pMG22 (ref. 30)), 8 (PTtgR5; pMG23 (ref. 30)) and 10 (PTtgR6; pMG24 (ref. 30)) base pair linkers between the TtgR operator (OTtgR) and the minimal promoter (PhCMVmin). Transfected HT-1080 were cultivated in the presence of different linoleic acid concentrations and SEAP expression was profiled after 48 h. Error bars indicate s.d. (n=4).