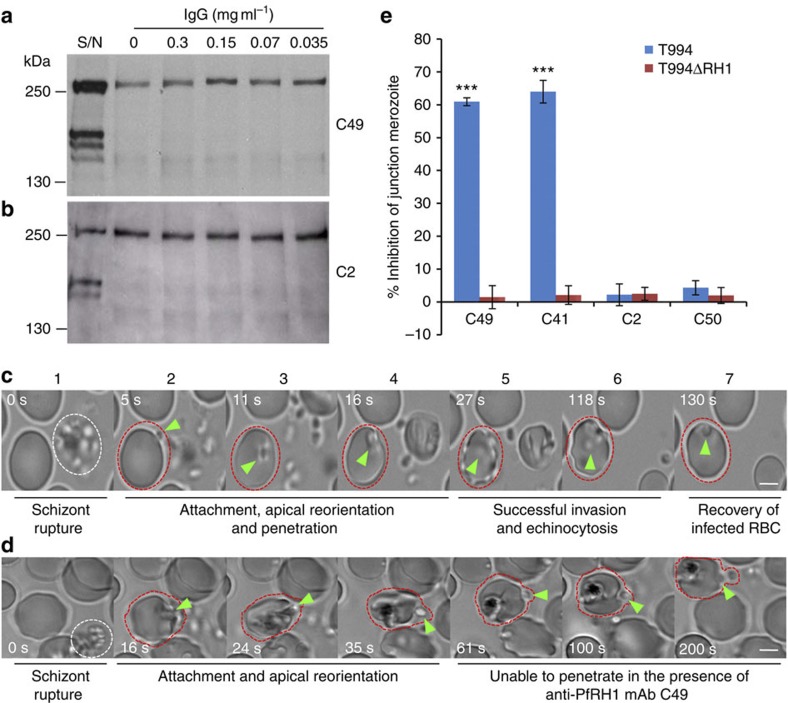
Figure 2. Anti-PfRH1 mAbs inhibit invasion before junction formation.
(a,b) Erythrocyte binding assays of T994 parasite culture supernatants using the C49 (a) and C2 (b) mAbs. Neither C49 nor C2 (0.035–0.3 mg ml−1) was able to inhibit the erythrocyte binding of PfRH1. T994 parasite culture supernatants (S/N) were used as a quality control. Molecular sizes are indicated on the left (in kDa). (c,d) Snapshots taken from time-lapse live movie microscopy of invasion of W2mef by merozoites in the absence (c) or presence (d) of C49 mAb. Time-elapsed post schizont rupture is indicated in each snapshot in seconds (sec, white). A white dotted circle indicates a rupturing schizont. Green arrows point to merozoites. A red dotted circle around an erythrocyte is added to help follow the infection. Scale bars=5 μM. (c) In the absence of mAb, the merozoite released from a mature schizont (white cycle) attaches, apically reorients and penetrates into an uninfected erythrocyte. Following successful invasion, deformation of infected erythrocytes occurs (echinocytosis) and by 2 min the erythrocytes recover back to its normal shape. (d) In the presence of C49, the merozoite released from a mature schizont attaches and apically reorients, but it is unable to penetrate the erythrocyte even after 3 min. Also see Supplementary Movies 1,2. (e) Anti-PfRH1 inhibitory mAbs significantly reduce merozoite junction formation. T994 or T994ΔRH1 schizonts were pretreated with Cyto D and allowed to rupture in the absence or presence of anti-PfRH1 mAbs (0.2 mg ml−1). Junction-arrested merozoites were counted microscopically. Bar chart indicates the counting of junction-arrested merozoites with mAbs C2, C41, C49 and C50 in T994 and T994ΔRH1 compared with the arrested parasites in the absence of mAbs. Inhibitory mAbs C49 and C41 significantly blocked merozoite junction formation in T994. The error bar indicates the s.e.m.; n=3. ***P≤0.00014 by one-way ANOVA indicates the significant differences between the effects of inhibitory mAbs (C49 and C41) and those of non-inhibitory mAbs (C2 and C50).