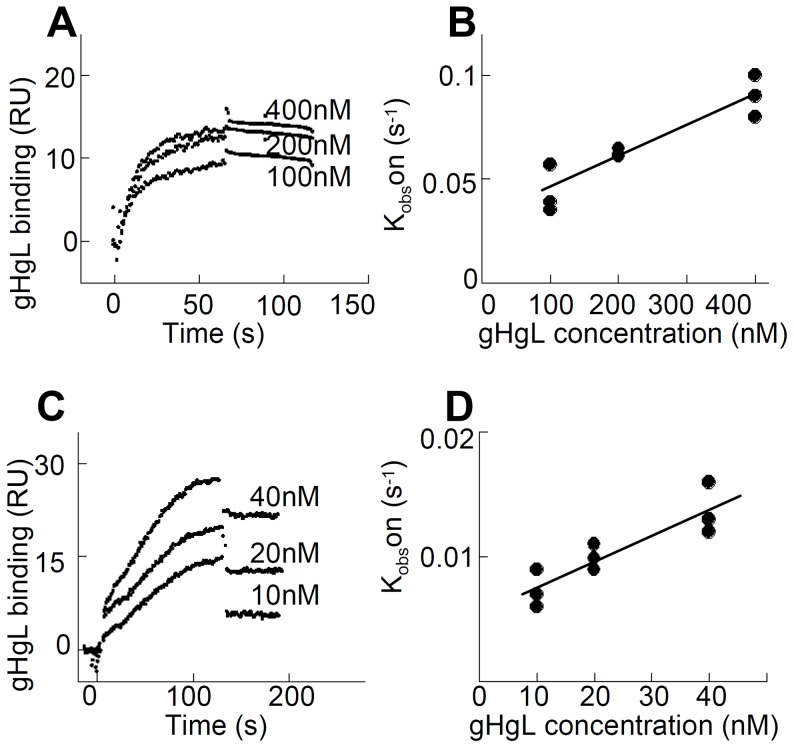
Figure 1. A soluble form of HSV gH/gL binds soluble αvβ6- and αvβ8–integrin at high affinity.
(A and C) Surface Plasmon Resonsance spectroscopy was performed to evaluate binding of different concentrations of gH/gL, as indicated, to immobilized αvβ6-integrin (A) and αvβ8-integrin (C). (B and D) Linear plots of k obs versus gH/gL concentration for the gH/gL interaction with αvβ6-integrin (B) and αvβ8-integrin (D). The intersection points with the Y axes correspond to the dissociation rates constants koff and the slopes to the association rate constants kon. Three independent experiments were performed for each integrin and results were plotted together without normalizing.