Abstract
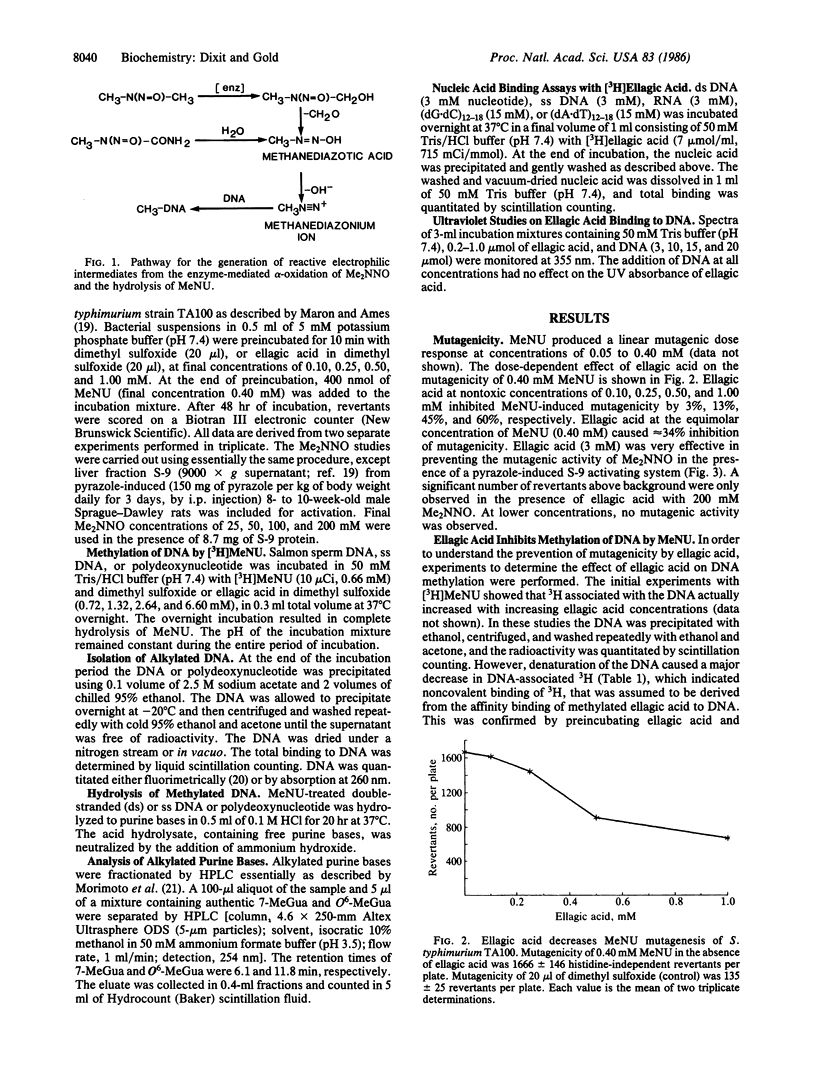
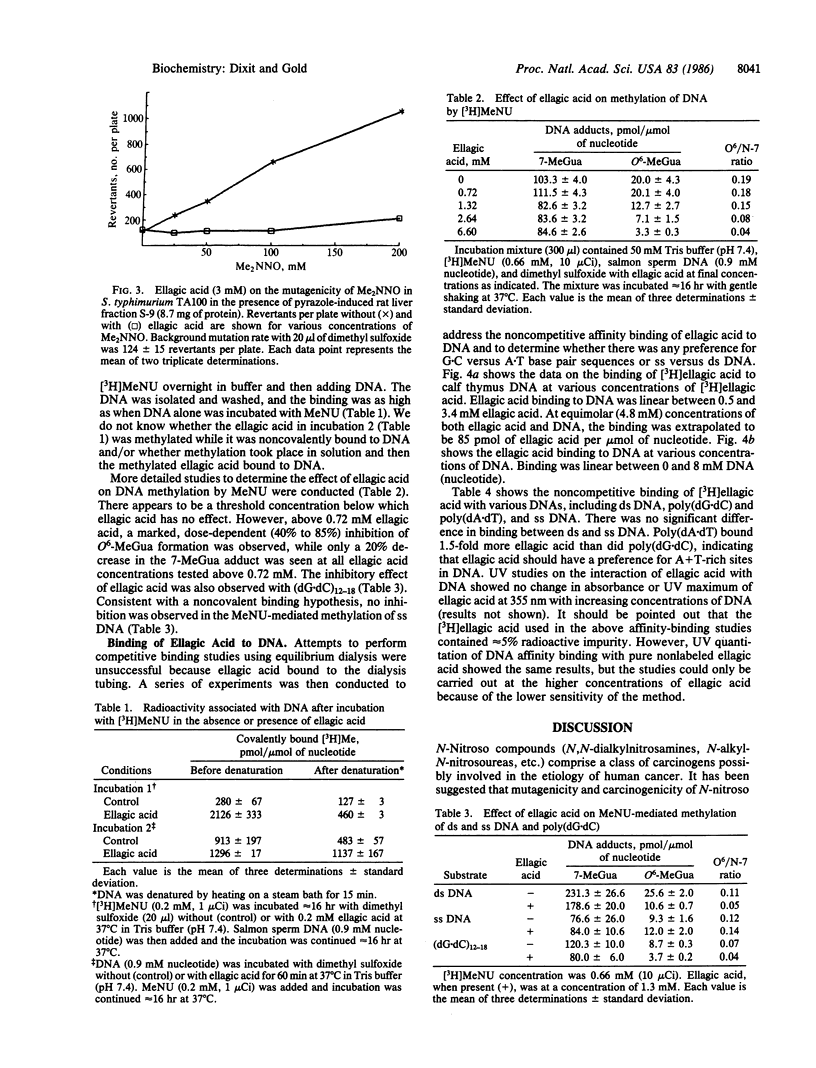
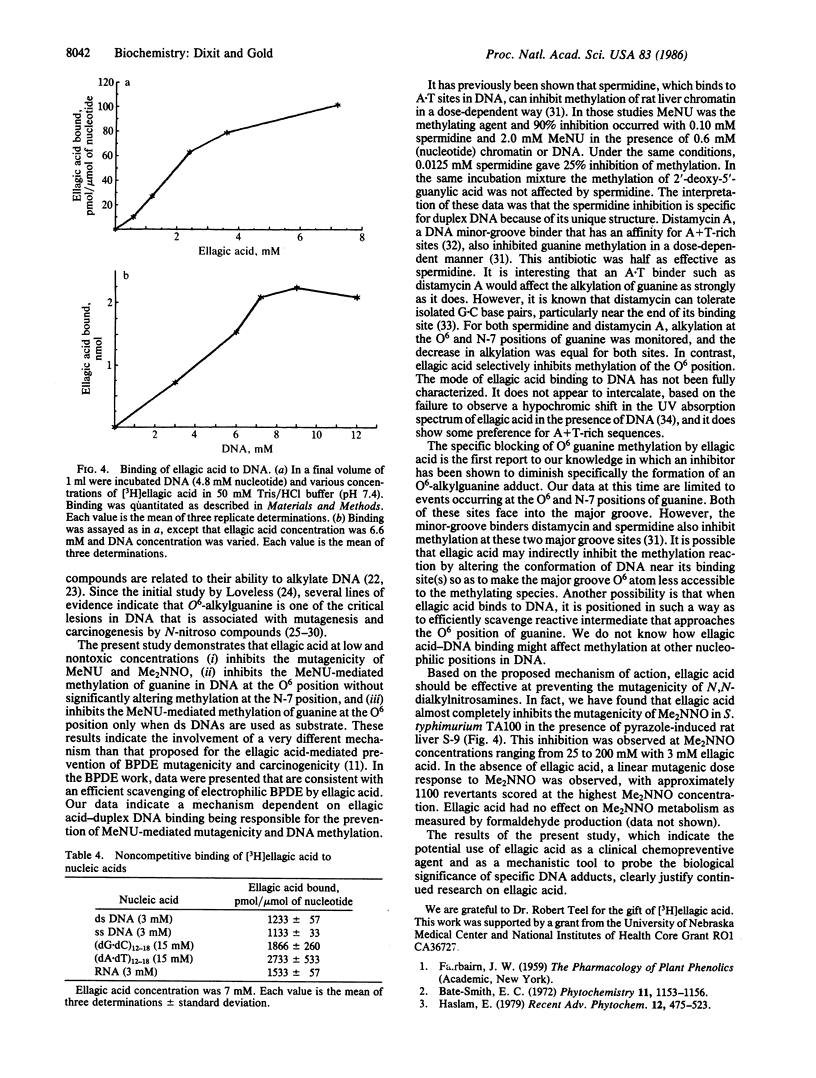
Ellagic acid, a naturally occurring plant phenol, inhibits the activity of the direct-acting mutagen N-methyl-N-nitrosourea (MeNU) in Salmonella typhimurium TA100. Ellagic acid at 0.10, 0.25, 0.50, and 1.00 mM inhibited the mutagenicity of MeNU (0.40 mM) by 3%, 13%, 45%, and 60%, respectively. Ellagic acid (3 mM) also inhibited the mutagenic activity of N,N-dimethylnitrosamine (25-200 mM) in the presence of pyrazole-induced rat liver fraction S-9. The effect of ellagic acid on DNA methylation was studied by incubating 0, 0.72, 1.32, 2.64, and 6.60 mM ellagic acid with DNA (0.9 mM nucleotide) and [3H]MeNU (0.66 mM). HPLC analysis of DNA hydrolysates showed that ellagic acid caused a dose-dependent 36-84% decrease in O6-methylguanine but only a 20% decrease in the 7-methylguanine adduct. Under conditions where methylation at the O6 position of guanine in double-stranded DNA was inhibited 65% by ellagic acid, no significant inhibition of either O6- or 7-methylguanine formation was detected in single-stranded DNA. Affinity-binding studies revealed that [3H]ellagic acid binds equally to double-stranded or single-stranded DNA but that poly(dA X dT) binds 1.5 times as much ellagic acid as does poly(dG X dC). The binding of ellagic acid to DNA is dependent on the concentration of both ellagic acid and DNA. The specific inhibition of O6-methylguanine formation only in double-stranded DNA and the relatively low inhibition of 7-methylguanine formation rule out the possibility that ellagic acid prevents DNA alkylation by scavenging the electrophilic intermediate generated in the hydrolysis of MeNU. The results suggest that ellagic acid inhibition of MeNU-induced mutagenicity is due to specific inhibition of methylation at the O6 position of guanine through an ellagic acid-duplex DNA affinity-binding mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers R. W., Sledziewska-Gojska E., Hirani-Hojatti S., Borowy-Borowski H. uvrA and recA mutations inhibit a site-specific transition produced by a single O6-methylguanine in gene G of bacteriophage phi X174. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7173–7177. doi: 10.1073/pnas.82.21.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. L., Huang M. T., Wood A. W., Wong C. Q., Newmark H. L., Yagi H., Sayer J. M., Jerina D. M., Conney A. H. Effect of ellagic acid and hydroxylated flavonoids on the tumorigenicity of benzo[a]pyrene and (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene on mouse skin and in the newborn mouse. Carcinogenesis. 1985 Aug;6(8):1127–1133. doi: 10.1093/carcin/6.8.1127. [DOI] [PubMed] [Google Scholar]
- Del Tito B. J., Jr, Mukhtar H., Bickers D. R. Inhibition of epidermal metabolism and DNA-binding of benzo[a]pyrene by ellagic acid. Biochem Biophys Res Commun. 1983 Jul 18;114(1):388–394. doi: 10.1016/0006-291x(83)91639-x. [DOI] [PubMed] [Google Scholar]
- Dixit R., Teel R. W., Daniel F. B., Stoner G. D. Inhibition of benzo(a)pyrene and benzo(a)pyrene-trans-7,8-diol metabolism and DNA binding in mouse lung explants by ellagic acid. Cancer Res. 1985 Jul;45(7):2951–2956. [PubMed] [Google Scholar]
- Doniger J., Day R. S., DiPaolo J. A. Quantitative assessment of the role of O6-methylguanine in the initiation of carcinogenesis by methylating agents. Proc Natl Acad Sci U S A. 1985 Jan;82(2):421–425. doi: 10.1073/pnas.82.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G., Pilbrow J. R. Physico-chemical probes of intercalation. Int J Biochem. 1984;16(12):1179–1192. doi: 10.1016/0020-711x(84)90215-5. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. L., Loechler E. L., Fowler K. W., Essigmann J. M. Construction and characterization of extrachromosomal probes for mutagenesis by carcinogens: site-specific incorporation of O6-methylguanine into viral and plasmid genomes. Proc Natl Acad Sci U S A. 1984 Jan;81(1):13–17. doi: 10.1073/pnas.81.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Chang R. L., Wood A. W., Newmark H. L., Sayer J. M., Yagi H., Jerina D. M., Conney A. H. Inhibition of the mutagenicity of bay-region diol-epoxides of polycyclic aromatic hydrocarbons by tannic acid, hydroxylated anthraquinones and hydroxylated cinnamic acid derivatives. Carcinogenesis. 1985 Feb;6(2):237–242. doi: 10.1093/carcin/6.2.237. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Wood A. W., Newmark H. L., Sayer J. M., Yagi H., Jerina D. M., Conney A. H. Inhibition of the mutagenicity of bay-region diol-epoxides of polycyclic aromatic hydrocarbons by phenolic plant flavonoids. Carcinogenesis. 1983 Dec;4(12):1631–1637. doi: 10.1093/carcin/4.12.1631. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesca P. Protective effects of ellagic acid and other plant phenols on benzo[a]pyrene-induced neoplasia in mice. Carcinogenesis. 1983 Dec;4(12):1651–1653. doi: 10.1093/carcin/4.12.1651. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Luck G., Zimmer C., Reinert K. E., Arcamone F. Specific interactions of distamycin A and its analogs with (A-T) rich and (G-C) rich duplex regions of DNA and deoxypolynucleotides. Nucleic Acids Res. 1977 Aug;4(8):2655–2670. doi: 10.1093/nar/4.8.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Morimoto K., Tanaka A., Yamaha T. Reaction of 1-n-propyl-1-nitrosourea with DNA in vitro. Carcinogenesis. 1983 Nov;4(11):1455–1458. doi: 10.1093/carcin/4.11.1455. [DOI] [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi S., Rao P. M., Sarma D. S. Studies on carcinogen chromatin--DNA interaction: inhibition of N-methyl-N-nitrosourea-induced methylation of chromatin--DNA by spermine and distamycin A. Biochemistry. 1978 Oct 17;17(21):4515–4518. doi: 10.1021/bi00614a024. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Huang M. T., Chang R. L., Newmark H. L., Lehr R. E., Yagi H., Sayer J. M., Jerina D. M., Conney A. H. Inhibition of the mutagenicity of bay-region diol epoxides of polycyclic aromatic hydrocarbons by naturally occurring plant phenols: exceptional activity of ellagic acid. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5513–5517. doi: 10.1073/pnas.79.18.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]


