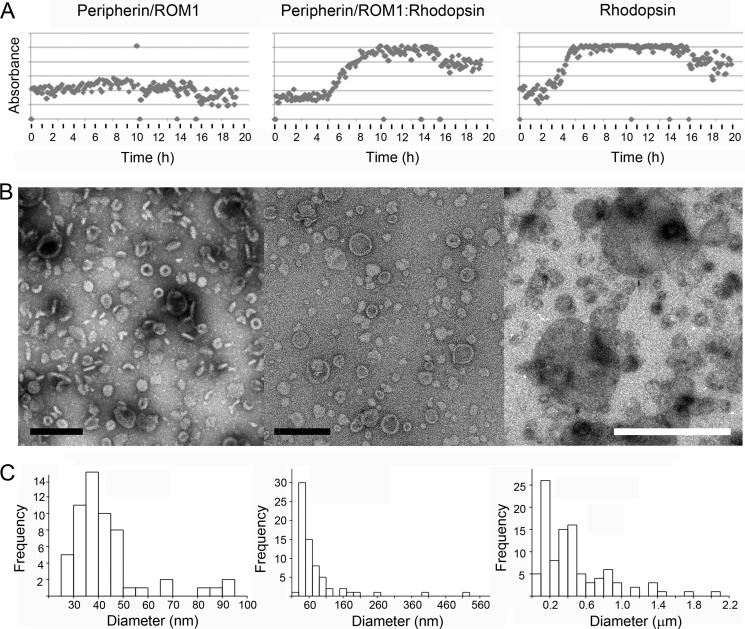
FIGURE 5.
Peripherin-ROM1 and rhodopsin incorporation into lipid vesicles. Purified peripherin-ROM1 complexes and/or rhodopsin were incorporated into protein-lipid aggregates by slow addition of methyl-β-cyclodextrin. A, light scattering is used to monitor incorporation of protein into vesicles. The transition from low to high scattering indicates formation of larger structures and is only observed in the presence of rhodopsin. B, micrographs of negatively stained reconstitution products. B, upon detergent removal, peripherin-ROM1 forms cylindrical rings. Scale bar: 200 nm. Upon addition of rhodopsin to the peripherin-ROM1-lipid mixture, larger discs with clear rims and rings are found. Scale bar, 200 nm. Rhodopsin alone reconstitutes into larger vesicles than in the presence of Peripherin-ROM1. Pronounced rims are missing. Scale bar, 1 μm. C, histogram showing distribution of vesicle diameters for the indicated proteins. Reconstitution conditions: peripherin-ROM1 (1 mg/ml), peripherin-ROM1-rhodospin (1:1, 1 mg/ml), or rhodopsin (1 mg/ml) were mixed with lipids at a lipid to protein ratio of 0.1 (w/w) in 100 mm NaCl, 5 mm MgCl2, 10 mm Bis-tris propane, pH 7. Fifty μm 11-cis-retinal was present in wells containing rhodopsin.