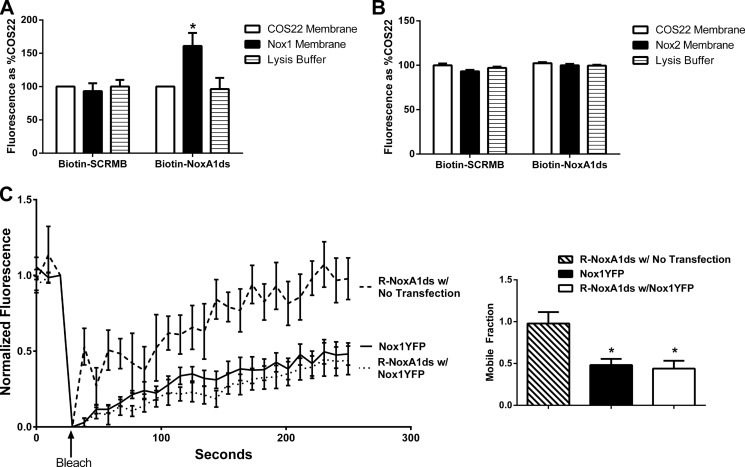
FIGURE 3.
NoxA1ds binds to Nox1 but does not detectably bind Nox2. A, neutravidin-coated plates were incubated with biotin-tagged NoxA1ds (Biotin-NoxA1ds) or biotin-tagged SCRMB (Biotin-SCRMB) before addition of cell membranes prepared from cells transfected with Nox1 (Nox1 membrane) or transfected with an empty vector (COS22 membrane). Bound Nox1 was detected through a FITC-conjugated secondary antibody bound to the Nox1 primary antibody. FITC fluorescence was expressed as binding as % COS22 membranes on each experimental day. There was no difference in binding between COS22 membranes and Nox1 membranes incubated with Biotin-SCRMB, whereas membranes incubated with Biotin-NoxA1ds showed a significant increase in binding when transfected with Nox1. n = 10–12, three separate experiments, *, p < 0.05, two-way ANOVA with Bonferroni post-test. B, neutravidin-coated plates were incubated with biotin-tagged NoxA1ds (Biotin-NoxA1ds) or biotin-tagged SCRMB (Biotin-SCRMB) before addition of cell membranes prepared from cells transfected with Nox2 (Nox2 membrane) or transfected with an empty vector (COS22 membrane). Bound Nox2 was detected through an Alexa 488-conjugated secondary antibody bound to the Nox1 primary antibody. Fluorescence was expressed as binding as % COS22 membranes on each experimental day. There was no difference in binding between COS22 membranes and Nox2 membranes incubated with Biotin-SCRMB or Biotin-NoxA1ds, C, FRAP of COS22 cells treated with 70 nm rhodamine B-labeled NoxA1ds (R-NoxA1ds) in the absence and presence of Nox1YFP transfection and FRAP of Nox1YFP alone. Panel to the right represents mobile fraction of these groups after 250 s. *, p < 0.05, one-way ANOVA with Bonferroni post test.