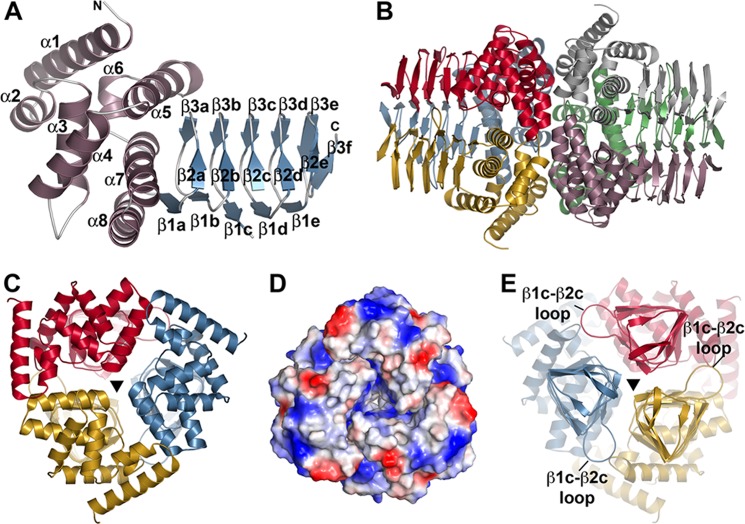
FIGURE 2.
Overall structure of GmSAT. A, monomer structure of GmSAT. The N-terminal α-helical domain and the C-terminal left-handed β-helix domain are colored rose and blue, respectively. Secondary structure features are labeled, as are the N and C termini. B, dimer of trimer arrangement of GmSAT. In the GmSAT apoenzyme and serine complex, crystallographic symmetry of chain A (red) and chain B (rose) complete the trimers shown on the left and right, respectively. C, view down the N-terminal 3-fold symmetry axis indicated by a triangle. D, surface electrostatic view of the N-terminal trimer, as in C. E, view (rotated 180° from that of C) down the C-terminal 3-fold symmetry axis indicated by a triangle. The β1c-β2c loop that forms part of the serine binding site is indicated.