Figure 6.
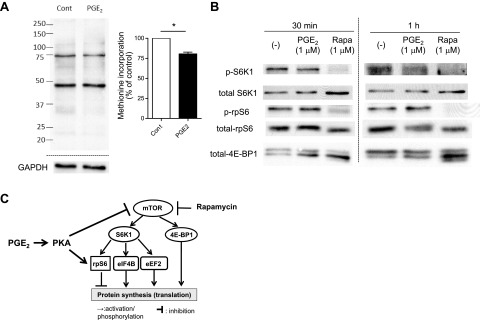
PGE2 inhibited global protein synthesis, suppressed the mTOR pathway, and enhanced activation of rpS6 in mouse PMs. PMs were harvested by lavage from naive C57BL/6 mice and were plated in serum-containing RPMI medium at 3–5 × 106 cells/ml for 2 h. A) PGE2-induced suppression of global protein synthesis in mouse PMs. After 2 h incubation in serum-containing medium, mouse PMs were washed twice with methionine- and serum-free medium and then were cultured for 1 h in the medium, with or without PGE2 (1 μM). Methionine analog AHA was added to each well at a final concentration of 25 μM, and cells were further incubated with AHA for 40 min. Then, cell lysates were harvested, and the Click-iT reaction was performed on the cell lysates to conjugate biotin to AHA analog incorporated into newly synthesized proteins. After electrophoresis of cell lysates, newly synthesized proteins containing biotin-conjugated AHA were detected with streptavidin-HRP. Left panel: representative immunoblot. Right panel: densitometry results from 3 experiments. Densitometry of the entire lane was expressed relative to that in control without any treatment (expressed as 100%, dashed line) in each experiment. *P < 0.05 vs. control without PGE2 treatment. B) PGE2 suppressed the mTOR pathway while enhancing activation of rpS6 in mouse PMs. After 2 h incubation in serum-containing medium, cells were washed twice with serum-free medium to remove nonadherent cells and then were incubated in the medium, with or without PGE2 (1 μM) or rapamycin (Rapa, 1 μM). At the indicated time points, cell lysates were harvested, and levels of phosphorylated S6K1 and rpS6 in the lysates were determined by immunoblot analysis. Immunoblot representative of 3 experiments is shown. C) Summary of the findings. PGE2, via PKA, activates rpS6 while suppressing the mTORC1 pathway; both actions contribute to inhibition of protein translation
