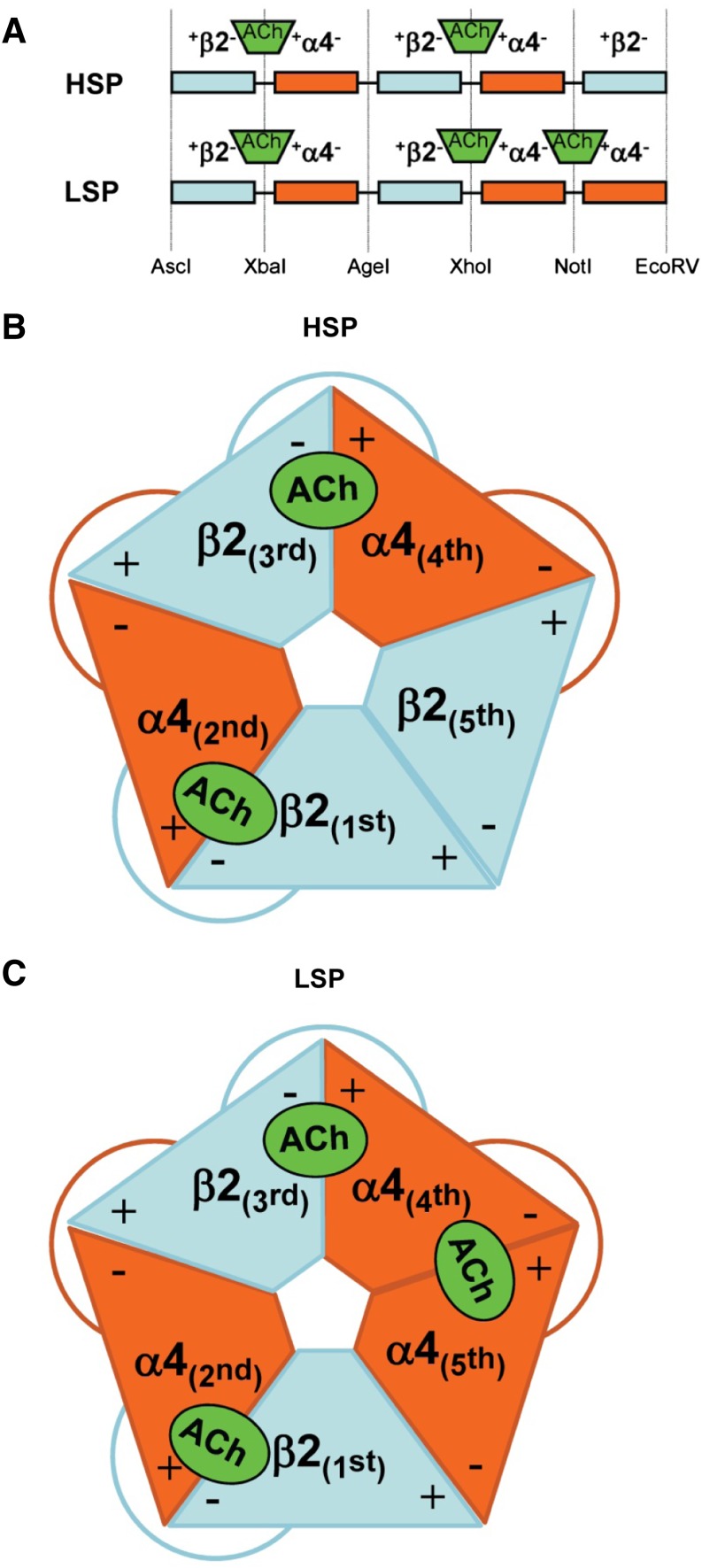
Fig. 1.
Schematic diagrams of concatenated HS and LS α4β2-nAChR isoform pentameric constructs (HSP and LSP, respectively). (A) At the cDNA level, individual subunits are joined using linkers that encode AGS repeats. Each linker also contains a unique restriction site, noted at the base of (A). These sites allow rapid removal and replacement of individual subunits by a simple restriction digestion and ligation approach (Carbone et al., 2009). For both HSP and LSP, long-recognized agonist binding pockets form at the interfaces between the principal (+) faces of α4 subunits in positions 2 and 4, and the complementary (−) faces of β2 subunits in positions 1 and 3, respectively. The functional pharmacology implications of the further, LSP-unique, agonist binding pocket between the (+) face of α4 position 5 and the (−) face of α4 position 4 are explored in this study; site-directed mutant α4 subunits were substituted in positions 4 and 5 of the original LSP construct. (B) Schematic of an assembled HSP α4β2-nAChR, showing the arrangement of the subunit + and – interfaces, the resulting pair of agonist binding pockets, and the linkers. (C) Schematic of an assembled LSP α4β2-nAChR. Note that much of the structure is similar to the HSP isoform, but that an α4 subunit is substituted for the β2 subunit in position 5. This gives rise to the additional agonist binding site shown between the fourth and fifth subunits of LSP α4β2-nAChR.