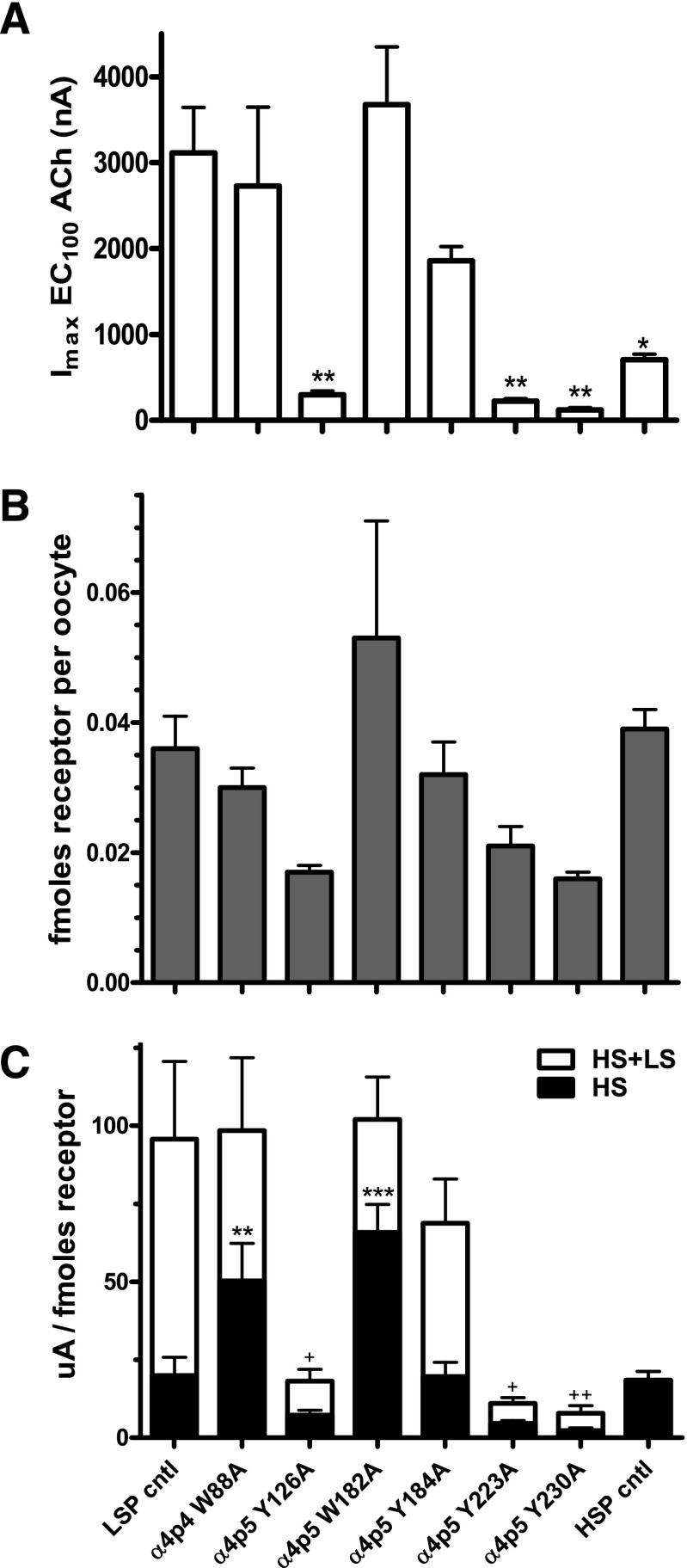
Fig. 8.
LSP α4(+)/(−)α4 interface mutation effects on maximum peak ACh-induced function (Imax) and nAChR cell-surface expression. X. laevis oocytes were injected with mRNAs encoding either unmodified or mutant LSP constructs (loop A, Y126A; loop B, W182A and Y184A; loop C, Y223A and Y230A; loop D, W88A), or an unmodified HSP construct (see Materials and Methods for details). (A) Maximum peak ACh-induced function (Imax) was determined for each construct. Imax is significantly reduced by several of the mutations compared with the unmodified LSP construct, and the HSP construct also has a significantly lower Imax value than that recorded for the wild-type LSP construct. *P < 0.05; ***P < 0.001. (B) nAChR protein expression at the surface of X. laevis oocytes was determined using an [125I]mAb295 binding assay. To note, binding of two [125I]mAb295 molecules per LSP construct and three per HSP construct (reflecting the different β2 subunit numbers in each nAChR isoform; see Materials and Methods) was assumed. Although some apparent variation in nAChR cell-surface expression was observed across the tested constructs, this did not reach the level of statistical significance. (C) Imax values were normalized to the amounts of nAChR cell-surface expression for each construct. By applying the HS-phase function fractions calculated for each construct (Table 1), it was possible to determine the absolute amount of HS- and LS-phase function for each construct. Significant changes are noted as follows: **P < 0.01; ***P < 0.001 for HS-phase function; and +P < 0.05; ++P < 0.01 for overall function (Imax). To note, the HSP result was not included in the overall function comparison for (C), since this construct produces only HS-phase function. Details of the analysis applied, and parameter values determined, are supplied in Table 2.