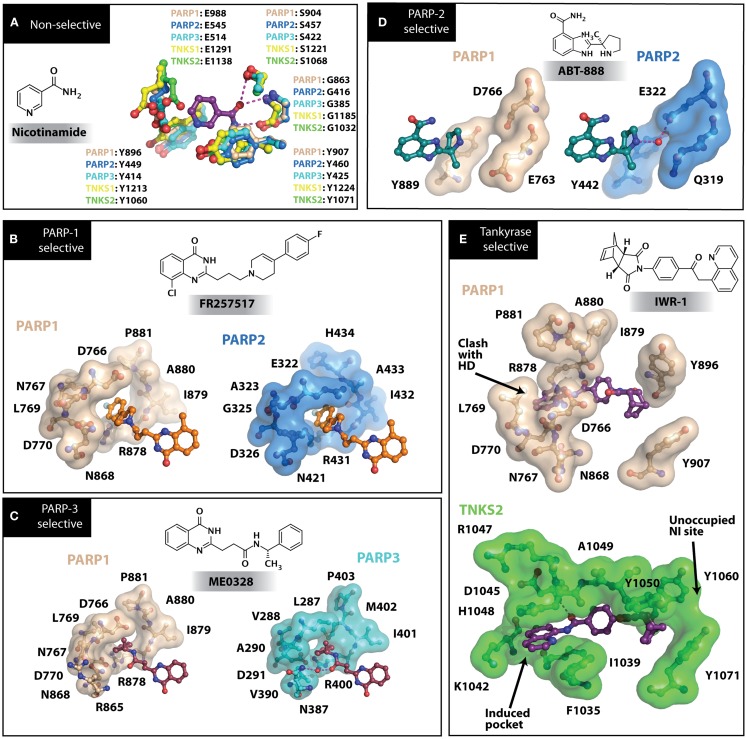
Figure 3.
PARP inhibitors bound to the catalytic domain of PARPs. Non-selective inhibitors such as nicotinamide [(A), non-selective] only interact with the nicotinamide pocket (NI site), which is a highly conserved region. Most developed PARP inhibitors have been designed to bind the NI site and adjacent sites to gain potency and selectivity. The compound FR257517 contains a fluorophenyl that reaches into the ADP-ribose binding site (AD site) of PARP-1 (PDB ID: 1UK0) to gain selectivity [(B), PARP-1 selective]. An aligned PARP-2 structure (PDB ID: 3KCZ) shows how the AD site is very similar to that of PARP-1, but the increased hydrophobicity of the PARP-1 AD site is attributed to the observed PARP-1 selectivity. Compounds that interact with E322 of PARP-2 (PDB ID: 3KJD) can gain selectivity over PARP-1 due to the differences in distance between this acidic side-chain and drug heteroatoms [(D), PARP-2 selective]. PARP-3 (PDB ID: 4GV4) has a structurally similar AD site as PARPs 1 and 2, although residue variation creates an environment distinct in polarity that guides selectivity [(C), PARP-3 selective]. Tankyrase inhibitors often demonstrate a much higher window of selectivity from PARPs 1–4, although selectivity between TNKS1 and TNKS2 is difficult to obtain. IWR-1 is a non-traditional PARP inhibitor in that it does not target the nicotinamide site of TNKS2 [(E), Tankyrase selective]. PARP-1 (PDB ID: 1UK0) was aligned with the co-crystallized TNKS2 structure containing IWR-1 (3UA9) to demonstrate that the quinoline ring clashes into the AD site of PARP-1 due to the presence of its helical domain.