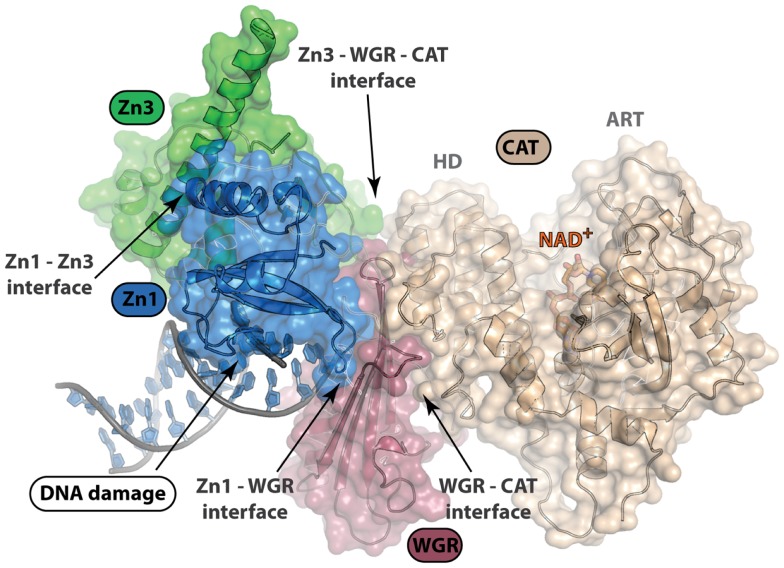
Figure 4.
Structure of PARP-1 in complex with DNA damage. PARP-1 binds DNA damage and activates catalytic activity nearly 500-fold. Only four of the six domains of PARP-1 (Zn1, Zn3, WGR, and CAT) are essential for DNA-dependent PARP-1 activation. This structure depicts complex formation and protein–protein interactions between domains upon DNA damage recognition (PDB ID: 4DQY). Disruption of these interdomain protein interfaces could be of interest in selective, allosteric targeting of PARP-1. An understanding of the arrangement of PARP-1 domain architecture in the absence of DNA damage recognition will be important for rational drug design efforts targeting protein interactions.