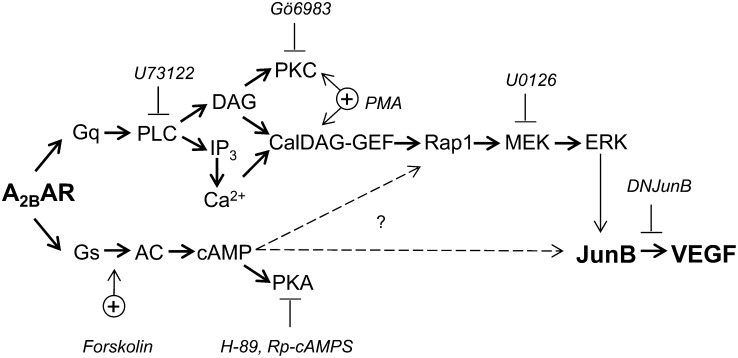
Fig. 9.
Schematic representation of A2B receptor-stimulated intracellular pathways involved in regulation of VEGF. A2B adenosine receptors (A2BAR) are coupled to adenylate cyclase (AC) via Gs proteins. Activation of this pathway results in accumulation of cAMP and stimulation of PKA. A2BAR are coupled also to PLC via a GTP-binding protein of the Gq family. Activation of this pathway results in accumulation of DAG and inositol trisphosphate (IP3), and the latter triggers mobilization of calcium from intracellular stores (Feoktistov and Biaggioni, 1995). In this study, we present evidence that A2BAR increase JunB protein levels and VEGF production via stimulation of PLC and ERK, which are possibly linked by the CalDAG-GEF–Rap1 pathway. PMA, an activator of PKC and CalDAG-GEF, also increased JunB protein levels and VEGF production. However, the broad-spectrum PKC inhibitor Gö6983 had no effect on the A2BAR-dependent increase in JunB protein levels and VEGF production. In contrast, the PLC inhibitor U73122 inhibited the A2BAR-dependent Rap1 activation, the increase in JunB protein levels, and VEGF production. The MEK inhibitor U0126 blocked the A2BAR-dependent stimulation of ERK and inhibited an increase in JunB protein levels and VEGF production. Stimulation of AC by forskolin also increased JunB protein levels and VEGF production. Broken arrows in the diagram signify potential effects of cAMP. These effects are PKA-independent because the PKA inhibitors with different mechanisms of action H-89 and Rp-cAMPS had no effect on the A2BAR-dependent increase in JunB protein levels and VEGF production. Finally, the overexpression of a DNJunB inhibited A2BAR-dependent increase in VEGF transcription and secretion, indicating an important role of JunB in this process.