Figure 2.
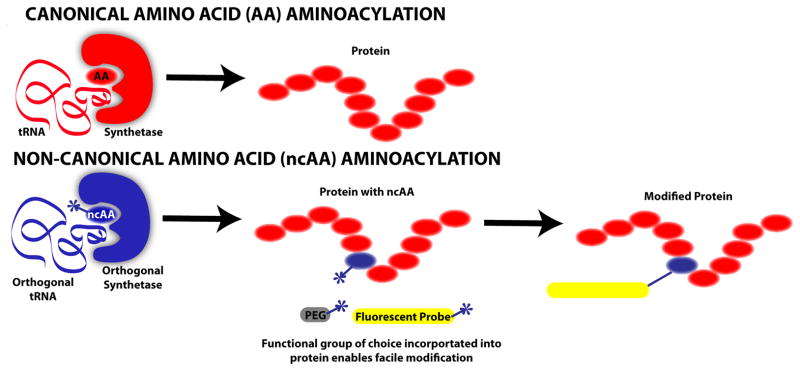
Incorporation of non-canonical amino acids (ncAA) into proteins. ncAA incorporation into proteins has been shown via multiple methods. The schematic above contrasts canonical amino acid (AA) incorporation into proteins with site-specific ncAA incorporation. Canonical AA incorporation proceeds following aminoacylation catalyzed by an endogenous aminoacyl-tRNA synthetase, which charges tRNA with a cognate amino acid. ncAA aminoacylation is possible via incorporation of a heterologous, orthogonal (i.e. not cross-reactive with host cell machinery) tRNA:synthetase pair into the cell responsible for protein production. Due to this incorporation, an orthogonal ncAA can be inserted site-specifically in response to a specific codon (typically a stop codon). A given ncAA typically has similar structure to a canonical AA except that a desired, atypical chemical functional group is included such that it is accessible to participate in a reaction. Thus, once translated, a protein incorporating a ncAA is readily modifiable with molecules such as polyethylene glycol (PEG), which improves circulation and limits immediate renal clearance, or with a molecule such as a fluorescent probe that facilitates imaging, as well as many other potential possibilities.
