Table 2.
Catalytic Asymmetric Synthesis of 1,1-Diarylalkanes from Racemic Benzylic Alcohols: Scope with Respect to the Alcohola
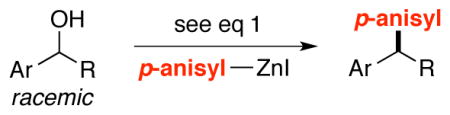
| ||||
|---|---|---|---|---|
| entry | benzylic alcohol | ee (%) | yield (%)b | |
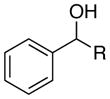
| 1 |
|
R =Me | 88 | 88 |
| 2 | Et | 94 | 92 | |
| 3 | n-Pr | 94 | 90 | |
| 4 | n-Bu | 95 | 83 | |
| 5 | i-Bu | 95 | 77 | |
| 6 | cyclobutyl | 94 | 51 | |
| 7c |
|
95 | 80 | |
| 8 |
|
88 | 90 | |
| 9 |
|
86 | 59 | |
| 10d |
|
94 | 77 | |
| 11 |
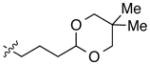
|
92 | 84 | |
| 12 |
|
94 | 84 | |
| 13 |
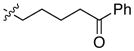
|
95 | 88 | |
| 14 |
|
94 | 75 | |
|
|
||||
| 15 |
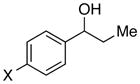
|
X = F | 93 | 87 |
| 16 | Cl | 90 | 88 | |
| 17 | Br | 91 | 79 | |
| 18 | Ph | 92 | 78 | |
| 19e |
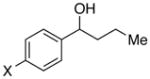
|
X = OMe | 95 | 94 |
| 20c,e | OPiv | 84 | 84 | |
|
|
||||
| 21e |
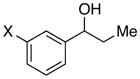
|
X = OMe | 94 | 94 |
| 22c | CF3 | 89 | 63 | |
| 23e |
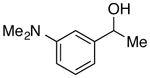
|
92 | 88 | |
|
|
||||
| 24 |
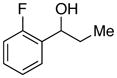
|
81 | 66 | |
| 25 |
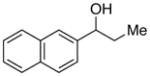
|
91 | 79 | |
| 26 |
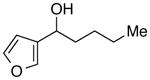
|
93 | 88 | |
| 27 |
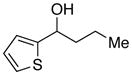
|
83 | 54 | |
| 28 |
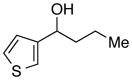
|
94 | 93 | |
All data are the average of two experiments.
Yield of purified product.
Reaction temperature: −35 °C.
Nucleophile: (p-tolyl)ZnI.
Nucleophile: PhZnI.
