Table 3.
Catalytic Asymmetric Synthesis of 1,1-Diarylalkanes from Racemic Benzylic Alcohols: Scope with Respect to the Nucleophilea
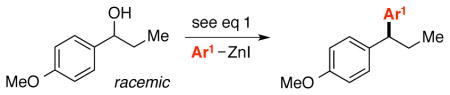
| ||||
|---|---|---|---|---|
| entry | Ar1 | ee (%) | yield (%)b | |
| 1 |
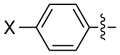
|
X = H | 94 | 96 |
| 2c | Me | 93 | 91 | |
| 3 | F | 94 | 95 | |
| 4 | Cl | 94 | 95 | |
| 5 | Br | 94 | 87 | |
| 6c | I | 94 | 66 | |
| 7 | CF3 | 94 | 98 | |
| 8d | CO2Et | 93 | 91 | |
| 9d | CN | 92 | 83 | |
|
|
||||
| 10 |
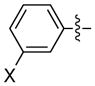
|
X = CF3 | 94 | 96 |
| 11d | CO2Et | 93 | 92 | |
|
|
||||
| 12 |
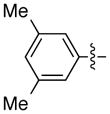
|
93 | 87 | |
| 13e |
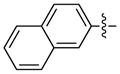
|
92 | 93 | |
| 14d |
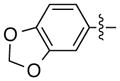
|
94 | 87 | |
| 15d |
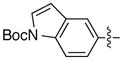
|
93 | 77 | |
All data are the average of two experiments.
Yield of purified product.
Equivalents of arylzinc reagent: 1.5.
Benzylic alcohol coupling partner: 1-phenylpropanol.
Reaction temperature: −30 °C.
