Abstract
The European honey bee (Apis mellifera) is a highly valuable, semi-free-ranging managed agricultural species. While the number of managed hives has been increasing, declines in overwinter survival, and the onset of colony collapse disorder in 2006, precipitated a large amount of research on bees' health in an effort to isolate the causative factors. A workshop was convened during which bee experts were introduced to a formal causal analysis approach to compare 39 candidate causes against specified criteria to evaluate their relationship to the reduced overwinter survivability observed since 2006 of commercial bees used in the California almond industry. Candidate causes were categorized as probable, possible, or unlikely; several candidate causes were categorized as indeterminate due to lack of information. Due to time limitations, a full causal analysis was not completed at the workshop. In this article, examples are provided to illustrate the process and provide preliminary findings, using three candidate causes. Varroa mites plus viruses were judged to be a “probable cause” of the reduced survival, while nutrient deficiency was judged to be a “possible cause.” Neonicotinoid pesticides were judged to be “unlikely” as the sole cause of this reduced survival, although they could possibly be a contributing factor.
Keywords: honey bees, causal analysis, neonicotinoids, Varroa
INTRODUCTION
The European honey bee (Apis mellifera) is a semi-free-ranging managed agricultural species, highly valued throughout the world for its production of honey and its ecological importance in plant reproduction, and increasingly, for pollination of many economically important crops. Although the worldwide number of managed hives has increased since the 1960s, declines in overwintering survival have been reported in the United States and in some European countries (Ellis 2012). Prior to 2006, annual average “expected” losses in the United States were approximately 15% (vanEngelsdorp et al. 2007), but since then, U.S. beekeepers have been reporting losses (overwintering mortality) of about 30% (vanEngelsdorp et al. 2011). These losses have not resulted in a pronounced decline in the overall number of honey-producing colonies managed in the United States, because beekeepers have apparently been replacing colonies to cover the losses (vanEngelsdorp et al. 2011). However, concern about these declines, which were perceived as widespread and substantial, as well as the onset of a particular phenomenon labeled colony collapse disorder (CCD; first described by vanEngelsdorp et al. 2007), has precipitated a large amount of research on bees' health over the past several years.
A number of possible causes for reduced overwinter survival of managed honey bees have been put forth in both the scientific literature and the popular media, including pests and parasites, bacteria, fungi, viruses, pesticides, nutrition, management practices, and environmental factors (vanEngelsdorp et al. 2010; vanEngelsdorp and Meixner 2010). Scientists are increasingly postulating that a combination of these factors is responsible (Kluser et al. 2010), and new hypotheses continue to surface. However, there is no consensus about the cause or combination of causes (vanEngelsdorp and Meixner 2010). A workshop of honey bee experts was convened in 2012 to use a formal causal analysis approach to elicit expert opinion and evaluate the possible causes of declining overwinter survival of honey bee colonies. Exponent, Inc. was contracted by Bayer CropScience to arrange and conduct the workshop.
CAUSAL ANALYSIS WORKSHOP
The goal of the workshop was to develop an organized framework to support an objective approach to evaluating the cause(s) of reduced overwinter survival of honey bees in the United States. The approach used a formal causal analysis approach to elicit and organize expert opinions. This method has been applied to various environmental problems where multiple causes were suspected (Suter et al. 2010; Wickwire and Menzie 2010). Using this systematic approach, the workshop participants identified and ranked the most important candidate causes of reduced overwinter survival and determined whether any of the candidate causes could be negated; critical data gaps and research needs that prohibited reaching a definitive conclusion were identified.
The causal analysis workshop was held at the Airlie Conference Center in Warrenton, Virginia, on September 25–27, 2012. The workshop participants included 19 honey bee experts chosen to represent a diversity of expertise in various factors affecting honey bee health, based on a literature search of relevant publications, and to represent the academic, industrial, and government sectors in North America and Europe. Areas of specialization included honey bees' biology and health, eco-toxicology, and beekeeping practices. The workshop was guided by causal analysis experts from Exponent; the U.S. Environmental Protection Agency (USEPA), Office of Research and Development; and the University of California Davis. Their areas of expertise include development of the USEPA causal analysis/diagnosis decision information system (CADDIS) and methods for exploring causation (e.g., change-point analysis, information theory, and expert elicitation) (http://www.epa.gov/caddis; Suter et al. 2010; Cormier et al. 2010; Liu et al. 2012; Martin et al. 2011; Moore and Runge 2012; Thomson et al. 2010). The honey bee experts were provided with an introduction to the causal analysis framework and were presented with an example of the approach, showing how it was used to identify the primary cause of the decline of the San Joaquin kit fox population in the southern Central Valley of California.
THE CAUSAL ANALYSIS FRAMEWORK
The causal analysis framework used for this exercise was adapted from the process developed by USEPA for causal assessments in impaired aquatic systems (CADDIS) (Suter et al. 2010; Cormier et al. 2010) and adaptations to terrestrial systems by Wickwire and Menzie (2010) and to evaluations of endangered species (Gard and Menzie 2012). The underlying approach and concepts have been used in applications related to environmental impacts on human health (Hill 1965) and more recently, in ecological impairment (USEPA 2000; Menzie et al. 2007). The causal analysis approach focuses on a specific individual case or problem and therefore is applicable to addressing the question of causation of reduced overwinter survival of honey bees.
A causal analysis framework leads to a supportable conclusion, because it is designed to (a) guard against gaps in logic concerning candidate causes and their effects, or prematurely identifying a singular cause; (b) provide transparency for professional judgments and scientific opinions; (c) identify principal causes; (d) distinguish among probable, possible, unlikely, and uncertain causes. The causal analysis method is intended to support expert judgments concerning the relative importance of each cause in decreased honey bees' survival and considers all major causes with a potential role in the problem. The causal analysis framework provides an outcome that categorizes the causes, based on the weight-of-evidence, as being probable, possible, or unlikely causes of declining honey bees' overwinter survival. The framework also helps identify data gaps and therefore can be used to guide the collection of additional data or novel research (Menzie et al. 2007).
As outlined by Wickwire and Menzie (2010), the causal analysis process includes the steps presented in Figure 1: (a) identifying the problem statement, (b) listing the candidate causes, (c) developing a conceptual model, (d) analyzing the strength of evidence compared to criteria, and (e) summarizing the significance of each cause.
Figure 1.
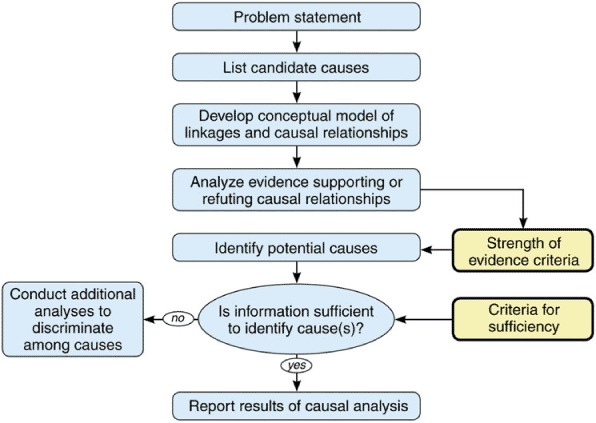
Causal analysis approach used for assessing reduced overwinter survival of honey bee colonies. (Color figure available online.)
PROBLEM STATEMENT
As a first step, the issue and problem statement was stated clearly, to identify the temporal and spatial bounds of the issue and the specific effect being studied. By placing bounds on the problem statement, the assessment became more tractable for a focused analysis. This was necessary because, without it, the problem was too broad and invited debate about its occurrence. For example, “global declines in honey bee populations” have been alleged (De Grandi-Hoffman et al. 2013) but lack rigorous scientific support. Therefore, it was decided among the workshop participants to focus on overwinter survival of honey bees managed for use in the California almond industry. There are nearly 740,000 acres of almond orchards in California that produce approximately 80% of the global almond market (Carman 2011), requiring honey bee pollination during the bloom period. The large pollination need for this industry has prompted U.S. beekeepers to move their colonies from states all over the country to provide contracted pollination services. It is estimated that nearly 60% of the bee colonies in the United States are used in pollination services for the California almond industry (Carman 2011). This scenario was therefore chosen due to its economic importance and the great number of colonies involved. The problem statement for this causal analysis was refined as follows:
What are the causes of the lowered probability of survival (on an annual‡ basis) of productive∗ commercial colonies of honey bees for almond pollination dating from approximately 2006 (recognizing preceding conditions)?
The date included in the problem statement reflects the fact that various factors have caused declines in honey bees in the past, but around 2006, higher-than-normal overwintering losses began to be reported (Ellis 2012). Although this time frame is also when the problem of CCD began to be recognized, the workshop participants specifically chose not to focus on CCD, due to the lack of clarity about the extent of this specific syndrome (Ellis 2012).
CAUSE IDENTIFICATION AND CONCEPTUAL MODEL
After agreeing on a focused problem statement, the next step was to create a list of candidate causes that influence the survival of honey bees. An initial list was generated from literature information and refined at the workshop through discussion with the honey bee experts (Table 1). The initial list included the most discussed and published causes—causes that may be linked to honey bees' health based on reports in popular media and those of particular concern to scientists and beekeepers but whose causal relationships are not currently known. At this initial stage, all candidate causes were included.
Table 1.
List of candidate causes.
| Stressor | Description | Reference |
|---|---|---|
| Viral diseases | ||
| Acute Bee Paralysis Virus (ABPV) and Chronic Bee Paralysis Virus (CBPV) | ABPV and CBPV are Aparaviruses, + single-stranded RNA viruses in the Dicistroviridae family. Both viruses cause uncontrollable trembling that prevents flight and causes paralysis. | EMBL-EBL (no date) |
| Black Queen Cell Virus (BQCV) | BQCV is a Cripavirus, a + sense, single-stranded RNA virus in the Dicistroviridae family. BQCV affects queen larvae, which die and turn black after the cell is sealed. | Oregon StateUniversity (2008) |
| Cloudy Wing Virus (CWV) | CWV is an icosahedral virus that causes the wings to become opaque due to crystalline structures of viral particles between muscle fibers; heavy infestation can cause mortality. | Biosecurity Authority Ministry of Agriculture and Forestry (2003) |
| Deformed Wing Virus (DWV) | DWV is an Aparavirus, + sense, single-stranded RNA virus in the Dicistroviridae family. DWV symptoms include vestigial and crumpled wings, bloated abdomen, paralysis, and severely shortened adult life span for worker and drone bees. DWV in combination with Varroa mites leads to immune suppression and subsequent disease from other pathogens. | Highfield et al. (2009) |
| Invertebrate Iridescent Virus (IIV-6) | IIV-6 is a double-stranded DNA virus in the Iridoviridae family. IIV-6 causes larvae to become inactive and die. | Bromenshenk (2011) |
| Israeli Acute Paralysis Virus (IAPV) | IAPV is an Aparavirus, a + single-stranded RNA virus in the Dicistroviridae family. IAPV causes wing tremors that progress to paralysis and then death outside of the hive. | Beeologics (no date) |
| Kashmir Bee Virus (KBV) | KBV is an Aparavirus, + single-stranded RNA virus in the Dicistroviridae family. KBV causes hairlessness, an oily appearance, an inability to fly, trembling, and eventual death. | British Columbia Ministry of Agriculture (2012) |
| Lake Sinai Virus 1 and 2 (LSV) | LSV I and 2 are RNA viruses discovered in 2011. | Runckel et al. (2011) |
| Sacbrood Virus (SBV) | SBV is an Iflavirus, + sense, single-stranded RNA virus in the Iflaviridae family. SBV infects larvae in the pre-pupa stage prior to cell capping and causes liquid to fill in the loose outer skin, resulting in death. | Agricultural Research Council (2010) |
| Varroa Destructor Virus 1 (VDV1) | VDV1 is an Iflavirus, a + sense, single-stranded RNA virus in the Iflaviridae family. VDV1 does not have a pathology description but appears to have the same effects as the Deformed Wing Virus. | Moore et al. (2011) |
| American foulbrood | Bacterial diseasesAmerican foulbrood is caused by Paenibacillus larvae subsp. Larvae, a rod-shaped, chain-forming bacterium. Larvae ingesting spores in their food can become diseased and die with as few as 35 spores. | Shimanuki and Knox (2000) and University of Georgia (2011) |
| European foulbrood (EFB) | European foulbrood is caused by Melissococcus plutonius, a short, non-spore-forming bacterium. These bacteria multiply and remain in the gut of larvae and compete for food, causing the larvae to die from starvation. | Shimanuki and Knox (2000) and Food and Environment Research Agency (2009) |
| Chalkbrood | Fungal diseasesChalkbrood is caused by the fungus Ascosphaera apis. Infected larvae rapidly reduce food consumption and then stop eating altogether. Infected larvae are covered by white fibrous mycelium, which fills the entire cell, and the larvae usually die after the cell has been capped. | Calderone (2001) and Aronstein and Murray (2010) |
| Nosema apis and Nosema ceranae | N. apis and N. ceranae are microsporidian fungi that invades the intestinal tracts of adults. Worker bees ingest spores when they are <1 week old and will not digest food well and are not capable of producing brood food secretions. Their lifespan is reduced by up to 78% and infected queens are superseded with a month. Bees are unable to leave the hive to eliminate waste, and dysentery develops. Nosema ceranae is replacing Nosema apis, even though its spores are less durable. | Runckel et al. (2011); Mussen (2011), Fries (2010) and Canadian Association of Professional Apiculturists (no date) |
| Stonebrood | Stonebrood is caused by the fungus Aspergillus (A. flavus, A. fumigatus, A. niger, and other species). Fungal mycelia penetrate the larvae and after death, the infected larvae become hardened. | Shimanuki and Knox (2000) |
| Crithidia | Pests and parasitesCrithidia are flagellate protozoan parasites found in the lumen or attached to the epithelium of the hindgut and rectum of adults. Their role in honeybee health is unclear but infestations in bumble bees during stressful conditions affect behavior and life span. | Shimanuki and Knox (2000), Evans and Schwarz (2011) |
| Greater Wax Moth (Galleria mellonella) and Lesser Wax Moth (Achroia grisella) | Due to its size, the Greater Wax moth is a more important pest. Adult wax moths do not affect the colony or spread disease, but wax moth larvae damage the comb by obtaining nutrients from honey, pollen, etc. | Mid-Atlantic Apicultural Research & Extension Consortium (2000), Shimanuki and Knox (2000), and Sanford (1995) |
| Phorid fly (Apocephalus borealis) | The phorid fly kills the bee upon emergence of the larva. It causes infected bees to become disoriented and stranded away from the hive. Phorid flies can be potential vectors for Nosema ceranae and DMV. | Core et al. (2012) |
| Small hive beetle (SHB) (Aethina tumida) | SHB does not affect the bee directly but affects the quality of the honey and in turn, affects the colony. Yeast from defecating SHB cause fermentation of the honey; the queen ceases laying, and the infested colony may be absconded. | Ellis and Eillis (2010), Zawislak (no date), and Ellis (2012) |
| Tracheal mites (Acarapsis woodi) | Tracheal mites are parasites that feed on honey bee hemolymph by piercing the tracheae. Bees die due to respiratory disruption from mites clogging the tracheae, from microorganisms entering the hemolymph, and from the loss of hemolymph. | Eishen (1987), USDA BARC (2002), and Sammataro et al. (2000) |
| Varroa mite (Varroa destructor) | Varroa mites are ectoparasites that feed on the hemolymph of immature and adult honey bees. Infected pupae do not develop into adults, and those that emerge have shortened abdomens, misshapen wings, deformed legs, and decreased weight. Varroa can transmit viruses such as DWV, ABPV, CBPV, SPV, BQCV, KBV, CWV, and SBV. | LeConte et al. (2012), Shimanuki and Knox (2000), and Ellis and Nalen (2010) |
| Environmental | ||
| Availability and quality of water | Limited access to water or access to contaminated water can affect honey bee health. | USDA (2012) |
| Bacillus thuringiensis (Bt) pollen | Bt is a gram-positive bacterium with Cry toxins that are used in insect-resistant genetically modified crops. Bt proteins in pollen can affect the hypopharyngeal gland in nurse bees and affect the ability to make brood food. | Rose et al. (2007) |
| Cell phones | Cell phones and cell phone towers have been linked to honey bee health in the popular media, originating from a small study in Germany that looked at whether a base station for cordless phones could affect honey bee homing systems. | USDA (2012) |
| Loss of feral populations resulting in reduced diversity of drones | A decrease in feral populations reduces the genetic pool from drones, and in turn, reduces the genetic variability of the queen's progeny. Loss of genetic variability can result in offspring with low genetic quality that are susceptible to disease and other effects. | NC State University (no date) |
| Reduction ofpropolis (saps and resins) | Propolis are resin and sap mixtures from plant sources used by honey bees to seal open spaces in the hive. Propolis is used to reinforce the structure of the hive, for protection against disease and parasites, and reduction in vibration. | Simone-Finstrom and Spivak (2010) |
| Sun spots | Sun spots are temporary events on the sun caused by intense magnetic activity. Sun spots cause disturbances in the earth's magnetic field, altering the honey bee orientation system for navigation. | Ferrari and Cobb (2010) |
| Weather events | Extreme weather events such as cold snaps and storms can result in colony loss, threaten nutritional success and ability to forage, and affect immunocompetence of bees already weakened by other factors. | Underwood and van Engelsdorp (2007) and Oliver (2010) |
| Beekeeping practices | ||
| Aggregation of hives in large agricultural situations | The overcrowding in apiaries as honey bees are transported to agricultural areas for pollination services induces stress and causes poor nutrition. | USDA (2012) |
| Antibiotics | Antibiotics are used to prevent honey bee disease (e.g., tetracycline is used for American foulbrood). However, resistance to tetracycline has prompted the use of new antibiotics like Tylosin. It is suspected that new antibiotics can affect the beneficial bacteria in honey bee guts. | Hathaway (2012) |
| Fungicides (in-hive) | Some classes of fungicides, such as ergosterol biosynthesis inhibitors, can inhibit cytochrome P450-mediated detoxification of pesticides. | Johnson et al. (2010) |
| Genetics (telomere premature aging syndrome) | Telomeres are protective DNA structures that provide a buffer for incomplete DNA replication during cell division in somatic cells. The gradual shortening of teleomeres may limit the lifespan of these somatic cells, causing impaired tissue regeneration and compromised immune systems. | Stindl and Stindl (2010) |
| Miticides (in-hive) | Miticides such as fluvalinate, coumaphos, and thymol are used to control Varroa infestations. Miticides can cause honey bee mortality, affect reproduction, and result in physical abnormalities and atypical behavior. Miticides can also interfere with the ability to properly integrate stimuli that elicit feeding, mating, colony defense, and communication behaviors. | Burley (2007), Haarmann et al. (2002), and Frost (2010) |
| Poor queens | Queens are often replaced when they are no longer productive. Due to genetics and poor health, poor queens can produce progeny with compromised health and less than desirable genetic diversity. | |
| Stress (e.g., transportation) | Stress, such as migratory stress during transportation, can compromise the immune system and increase disease susceptibility. | Johnson (2010) |
| Pesticides | ||
| Fungicides (external) | Fungicides are used to protect agricultural crops from fungal infections. For example, pyrethroids, organophosphates, carbamates, DDT, lidinale, etc. Neonicotinoids area sub-group of insecticides. | |
| Insecticides (external) | Insecticides used on crops to prevent insect damage may have effects. For example, pyrethroids, organophosphates, carbamates, DDT, lindate, etc. Neonicotinoids are a sub-group of insecticides. | |
| Neonicotinoids | Neonicotinoids are systemic neuro-active insecticides that can cause behavioral changes, reduce foraging activity, and increase foraging flight distance, and can cause acute mortality to honey bees at high doses. | Schneider et al. (2012) |
| Nutrition | ||
| Nutrition deficit (quality of food) | Pollen is a protein source and nectar is a carbohydrate source. The diversity and quality of food can affect a colony's number of pollen foragers. | Pernal and Currie (2001) |
| High-fructose corn syrup (HFCS) | HFCS is used in supplemental feeding. Problems associated with HFCS include toxicity due to hydromethylfurfural (HMF) if improperly stored. | Pernal and Currie (2001) and Alaux et al. (2010) |
| Starvation (quantity of food) | Nutrition is related to the proximity of the hives to available foods. The lack of adequate nutrition is partly a management issue. The lack of pollen resources just prior to winter may lead to immunosuppression, affect brood-rearing capacities, and decrease preparation for overwintering. | Mattila and Otis (2007) |
A conceptual model of how the candidate causes affect bees' survival was developed based on the honey bees' life cycle. The conceptual model characterizes the honey bee's biological characteristics and ecological requirements to assist in identifying its vulnerabilities to the candidate causes. The conceptual model also illustrates the relationships between the honey bees' life stages and each cause, as well as among the various candidate causes. If known, the relative magnitudes of the candidate causes on the life stages are also identified, such as indicating reproductive effects versus mortality effects. If known and recognized, causal relationships between candidate causes and the honey bee were also depicted in the conceptual model.
Causal Criteria
The causal criteria used to evaluate the strength of evidence for how each candidate cause reduces survival of honey bees used in the California almond industry scenario are described below:
Evidence of time order: A candidate cause must always precede or coincide with the event of concern. Temporality is an important consideration and should include knowledge of the life history of the species (e.g., seasonality) to determine whether the cause occurred just prior to a critical life stage, as well as more broadly, such as a few years prior to the problem. For this causal analysis, the decline in probability of survival for honey bees in the California almond scenario appeared to have started sometime around 2006. Candidate causes that were known to have occurred just prior to 2006 (or emerged at that time) provide stronger evidence of possible causality. Causes that were documented only after 2006 were given little further consideration in the assessment, because the evidence is considered weak.
Co-occurrence: To cause a problem, the candidate cause must have interacted both spatially and temporally with managed honey bee colonies. This criterion does not require physical contact between the cause and the effect and also includes the absence of the factor (e.g., lack of water and food sources). Time lags are considered, because some causes may have always been present but may not have been reported until later.
Cause–response relationship: This criterion is meant to describe the relationship between the candidate cause and its intensity, frequency, and duration. It is intended to determine whether the observed magnitude of the effect is concordant with the amount of the causal agent present in the environment. These relationships can be defined in the laboratory or in the field. Sufficiency in the laboratory can be elucidated from controlled experiments that have been performed to examine responses at various levels of stress. A classic example is dose and response testing of chemicals on test organisms. Sufficiency in the field relates to observed effects seen in field-scale experiments or reports from commercial beekeepers.
Interaction: The candidate cause must have a known (or hypothesized) mechanism of action that can result in the observed effect. This criterion is important in cases where the candidate cause and effect are spatially and temporally coincident but there is little information about the nature of the connection between the two.
Alteration: The candidate cause should result in some alteration to the honey bee that is notable, either decreased survival or some sublethal effect that has the potential to cause colony failure. The more specific the symptoms are to the candidate cause, the stronger is the weight-of-evidence that supports its diagnosis.
Scoring
The impact of each candidate cause and the relative strength of its association to the observed effect (overwinter survival of honey bees) was evaluated by the experts. A scoring system was used to assign the candidate cause to a category for each of the criteria described above, based on the degree of confidence expressed by the experts (USEPA 2008; USEPA CADDIS). Categories were designated by a range of “+” and “−” scores, as follows:
+++ convincingly supports
++ strongly supports
+ somewhat supports
0 neither supports or weakens
− somewhat weakens
− − strongly weakens
− − − convincingly weakens
NE no evidence
After each candidate cause was evaluated for each criterion, the body of evidence was evaluated for consistency by reviewing the overall pattern of “+” and “−” scores. A cause with many “++” and “+++” for multiple criteria was deemed to be a “probable cause” of reduced commercial honey bees' survival in the California almond scenario. In contrast, causes with a multitude of “− − −” and “− −” scores were considered to be unlikely causes (Table 2).
Table 2.
Weight-of-evidence for candidate causes and consistency of evidence.
| Cause-response relationship |
|||||||
|---|---|---|---|---|---|---|---|
| Candidate cause | Time order | Co-occurrence (Spatial and temporal) | Sufficiency (laboratory) | Sufficiency (field) | Known mechanism of action | Alteration | Conclusions of consistency of evidence |
| Viral diseases | |||||||
| ABPV + KBB + IAPV | − | + | +/0 | + | + | 0 | Unlikely |
| BQCV + SBV +CBPV +LSV 12 | 0 | + | 0 | 0 | 0 | 0 | Indeterminate |
| DWV in combination with other causes | ++ | ++ | + | + | ++ | Possible / contributor | |
| IAPV | + | Possible / contributor | |||||
| IIV–6 CWV VDV1 | − | Indeterminate | |||||
| Varroa Mites + virus | +++ | +++ | +++ | +++ | ++ | ++ + | Probable |
| Bacterial diseases | |||||||
| American foulbrood | − | − | + | − | + | — | Unlikely |
| European foulbrood | − | − | − | Unlikely | |||
| Fungal diseases | |||||||
| Chalkbrood | − | 0 | − | Unlikely | |||
| Nosema apis | − | + | + | + | Unlikely alone / contributor | ||
| Nosema ceranae | + | + / – | − | + | 0 | Unlikely alone / contributor | |
| N. apis + N. ceranae | + | − | + | + | Unlikely alone / contributor | ||
| Stonebrood | − | − | − | − | + | − | Unlikely |
| Pests and parasites | |||||||
| Crithidia | 0 | + | 0 | 0 | 0 | 0 | Indeterminate |
| Phorid fly | 0 | − | 0 | 0 | 0 | Unlikely | |
| Small hive beetle | − | − | Unlikely | ||||
| Tracheal mites | − | − | + | − | + | NE | Unlikely |
| Varroa destructor | +++ | +++ | + | + | ++ | +++ | Possible |
| Wax moth | − | − | Unlikely | ||||
| Environmental | |||||||
| Availability and quality of water | Indeterminate | ||||||
| BT pollen | + | + | − | − | − | − | Unlikely |
| Cell phones | + | − | − | − | Unlikely | ||
| Loss of feral populations reducing diversity of drones | + | 0 | 0 | 0 | + | 0 | Indeterminate |
| Reduction of propolis | Indeterminate | ||||||
| Sun spots | − | − | − | Unlikely | |||
| Weather events (e.g., cold snaps, rain, or timing of warming) and availability to forage | − | ++ | ++ | + | Unlikely alone / contributor | ||
| Pesticides | |||||||
| Fungicides (external) | + | − | + | + | + | 0 | Indeterminate |
| Insecticides (external) | ++ | − | + | + | + | Indeterminate | |
| Neonicotinoids | + | − | + / – | − | + | 0 | Unlikely alone / contributor |
| Beekeeping practices | |||||||
| Aggregation of hives in large ag | ++ | Indeterminate | |||||
| Situations | |||||||
| Antibiotics | + | 0 | Indeterminate | ||||
| Fungicides (in-hive) | + | − | + | + | + | 0 | Indeterminate |
| Genetics (e.g., telomere premature aging syndrome) | Indeterminate | ||||||
| Miticides (in-hive) | + | + / – | + | + / – | + | 0 | Possible / contributor |
| Poor queens | + | + | + | + | + | 0 | Possible / contributor |
| Stress (transportation) | ++ | Indeterminate | |||||
| Nutrition | |||||||
| Nutrition deficit | + | + | + | + | + | Possible | |
| Problems with supplemental feeding (HMF) | 0 | + | + | 0 | + | Indeterminate | |
| Starvation | + | + | + | ++ | + | + | Possible |
Due to time limitations, scoring was incomplete for some causes. Blank cells indicate that no score was assigned. “+/−” indicates disagreement among workshop participants.
Consistency of Evidence
To assist in organizing the data and interpreting the evidence, a weight-of-evidence table was created (Table 2). The causes were determined to be probable, possible, unlikely, or indeterminate. These categories are defined as:
Probable: There is convincing evidence that the cause has led to a decline in the survival probability of commercial honey bee colonies in the California almond industry, is impeding the recovery, and may cause further decline and/or continue to impede recovery.
Possible: There is some convincing evidence that the cause, acting alone, could contribute to the decline in survival probability of commercial honey bee colonies in the California almond industry. There is some evidence that the cause can contribute to a negative change in the population at the hive or colony level.
Possible in combination with other factors: There is some convincing evidence that the cause, in combination with one or more other causes, could contribute to a decline in the survival probability of commercial honey bee colonies in the California almond industry. There is weak evidence that the cause, acting alone, can contribute to a negative change in the population at the hive or colony level.
Unlikely: The evidence is too small or runs counter to the criterion that the cause can elicit a decline in the survival probability of commercial honey bee colonies in the California almond industry. Consistent negative evidence can be used to eliminate a potential cause.
Indeterminate: The evidence for the cause is not available, or more information and data are required to assess its effects on the survival probability of commercial honey bee colonies in the California almond industry.
RESULTS
Due to time limitations, workshop participants were unable to perform a detailed evaluation of the evidence for (and against) each candidate cause, and reaching a final conclusion is beyond the scope of this article. However, several examples are provided below to illustrate the causal analysis approach and the manner in which a weight-of-evidence argument can be developed to support a final conclusion. The evidence cited is not exhaustive but indicates the types of information that can be used in the analysis. An example is provided for causes that the workshop participants rated in each of the following three categories: probable cause, possible cause, and unlikely cause. These examples were selected to represent three different categories of conclusions as well as three different major categories of stressors (Pests and Parasites; Pesticides; and Nutrition).
Example of a Probable Cause
The combination of the parasitic mite Varroa destructor plus viruses received the highest ranking and was judged to be a “probable cause” of reduced honey bees' survival, as described in the problem statement (Table 2). Either Varroa or viruses occurring separately were felt to be “possible” causes, but the combination was assessed to be “probable.” Varroa destructor (formerly known as Varroa jacobsoni) is a mite that feeds on the hemolymph of both immature and adult honey bees. If not controlled, it is devastating to beekeepers in most parts of the world (ISSG 2006). Rosenkranz et al. (2010) stated that, without periodic treatment for Varroa, most of the honey bee colonies in temperate climates would collapse within 2–3 years. The following effects of Varroa on honey bees have been reported: decreased weight and lifespan of adult bees; severe nutritional deficits for the developing bee; malformations (shortened abdomens, misshapen wings, deformed legs); decreased flight duration, and reduced sperm production in drones (which at the colony level can result in reduced mating opportunities and swarms); increased absences and lower rate of return to the hive (possibly due to altered learning and navigation ability); reductions in emergence weights, water, protein, and carbohydrate levels (which may affect navigation and also compromise immune response); and reductions in indicators of long-term survival and endocrine function (Amdam et al. 2004; Bowen-Walker and Gunn 2001; LeConte et al. 2012; Ellis and Zettle Nalen 2010; Rosenkranz et al. 2010; Sammataro 2012; Duay et al. 2003; Garedew et al. 2004; DeJong 1997).
In addition, Varroa can transmit multiple viruses as well as activate dormant viruses, and it is increasingly believed that the viruses, not the mites, may be responsible for the majority of damage that bees experience while hosting the mites (Webster and Delaplane 2001; Hung et al. 1995, 1996). Bee viruses were considered a minor problem before the occurrence of Varroa (Rosenkranz et al. 2010), although viral disease outbreaks in colonies infected with Varroa inevitably result in the collapse of the colony (Allen and Ball 1996; Ball and Allen 1988). Transmission by Varroa has been proven experimentally for several honey bee viruses, as summarized by de Miranda et al. (2012). The list of viruses vectored by Varroa destructor includes ABPV, KBV, DWV, IAPV, VDV-1, SBPV, and SBV (Sammataro et al. 2000; Rosenkranz et al. 2010; de Miranda et al. 2012). This is supported by specific laboratory and field evidence (Ball and Allen 1988) for APV. Yang and Cox-Foster (2007) found that a combination of Varroa, the interaction of DWV and microbes, and a developmental immune incompetency decreased survivorship and colony fitness.
V. destructor is a new parasite of A. mellifera, as it has shifted from its original host (A. ceranae). Thus, the natural, relatively benign evolutionary compromise between bee viruses and their hosts has been seriously destabilized (de Miranda et al. 2012). The first record of Varroa in the United States dates to 1987 (DeJong 1977) and it is present on all continents except Australia (Rosenkranz et al. 2010). Thus, its appearance pre-dates the advent of the declines in honey bees' survivorship, as described in the problem statement, which began to occur around 2006. In addition, Varroa do not occur in Australia, a continent that has not experienced similar declines in honey bees' survival. Colony losses have not been significant in Africa and South America either, where African and Africanized bees, respectively, exhibit high survival without treatment for Varroa (Cobey et al. 2012). These points thus resulted in strong positive scores in the causal criteria for time order and co-occurrence.
The impacts of Varroa infestation on colony health are well documented. Although the relationship between the level of infestation (cause) and the damage produced (response) is complex (Rosenkranz et al. 2010), the level required to produce damage appears to have decreased over time (vanEngelsdorp and Meixner 2010). The effects of viruses on bees are becoming better understood. With experimental evidence from laboratory and field-scale studies that Varroa can transmit viruses (de Miranda et al. 2012), the causal criteria regarding the cause–response relationship and interaction (mechanism of action) can be judged to be convincingly or strongly supportive. The causal criterion of specific symptoms experienced by colonies suffering from the combination of Varroa and viruses was also judged to be convincingly supportive. These symptoms include reduced colony development, the presence of malnourished, deformed, and underweight bees, or crawling bees that are unable to fly or that have crippled wings.
The evidence for Varroa acting alone as a cause of low honey bees' survival is less compelling, probably because it is difficult to separate the effects of viruses from those of the mites. Thus, while Varroa destructor was rated as a possible cause, when the impact of viruses was added, this became a probable cause.
Observations suggest that infection with a contagious agent is responsible for colony loss (vanEngelsdorp et al. 2009; Cornman et al. 2012; Cox-Foster et al. 2007), which lends further support to viruses transmitted by Varroa as the proximate cause of honey bees' death and colony loss.
Example of a Possible Cause
The health of a colony depends on the presence of well-nourished bees that are capable of producing progeny and resisting multiple stress factors (Brodschneider and Crailsheim 2010). There are two issues related to food for bees. Nutrition deficiency is associated with the quality of the food. The second issue is starvation and the lack of availability of food, such as over winter. The example presented here as a possible cause of reduced colony survival is nutrient deficiency (Table 2).
Honey bees require a diverse diet that includes protein, carbohydrates, lipids, vitamins, minerals, and water for maintaining good health (Huang 2011). Carbohydrates from the sugars in nectar and honey are used as an energy source. Sugar content in nectar can vary from 5% to 75%, depending on the plant species (Huang 2011). Pollen provides a source of protein, minerals, lipids, and vitamins (Herbert and Shimanuki 1978). The nutritional value of pollen varies among plant species and regions (Brodschneider and Crailsheim 2010). Due to the variable nutrients in pollen and nectar, honey bee colonies can experience a deficiency in one or more nutrients if they are visiting the wrong plants—blueberries are such an example (vanEngelsdorp et al. 2007). Nutrient deficiency can lead to reductions in adult survival, including decreased lifespan of worker bees, compromised brood development, and subsequent depopulation of the colony (Naug 2009).
Nutrient deficiency has an indirect effect on adults’ survival. Sufficient food stores are required for adults to feed larvae and rear the larvae into adulthood. An inadequate store of pollen can affect the quality of the next generation of adults, and thus, can influence the rearing of subsequent brood. Larvae that are reared during nutrient-deficient periods have a reduced lifespan as adults or develop into adults with impaired brood rearing or foraging abilities (Brodschneider and Crailsheim 2010).
There is experimental evidence that a lack of nutrients in honey bees' diets is linked to immunocompetence. Eischen and Graham (2008) found that well-nourished honey bees are less susceptible to Nosema cerane, in comparison to less well-nourished bees. In another controlled study, bees that were fed a polyfloral diet of mixed pollen had enhanced immune functions, such as glucose oxidase activity, leading to better in-hive antiseptic protection. Glucose oxidase activity allows bees to sterilize colony and brood food (Alaux et al. 2010). The results suggest that there is a link between nutrition and immunity and underscores the critical role of resource availability to bees' health.
Nutrition is related to the proximity of the hives to available food. Evaluation of the co-occurrence criterion would indicate that, for our problem statement, it would be reasonable to expect that a lack of adequate nutrition is partly a management issue. Due to the relationship of nutrient deficiency and immunocompetence in bees, specificity in response is low, because other factors can result in similar responses. The weight-of-evidence evaluation indicates that there are data and information that somewhat support nutrient deficiency as a possible cause of the decreased survival of bees used to pollinate California almond orchards. Although nutrient deficiency can impair adult survival and lead to lack of immunocompetence, this cause does not translate well into reduced overwinter survival, and more research is needed to establish a causative link.
Example of an Unlikely Sole Cause
Neonicotinoid pesticides were considered to be an unlikely sole cause of reduced honey bee overwintering survival, although some of the experts thought they may be a contributory cause (Table 2). This class of pesticides includes clothianidin, imidacloprid, thiamethoxam, and thiacloprid. These compounds are systemic (e.g., are taken up throughout the plant tissues), and concern has been raised that bees can be exposed through pollen and nectar.
Imidacloprid was the first of the neonicotinoids to be approved for agricultural use (in 1994), and since then, its use in the United States, as well as that of other neonicotinoids, has increased greatly (Cresswell et al. 2012). Thus, these potential causes precede the decline in honey bees' survival, although the increased use between 1994 and 2005 did not precipitate such a response. Therefore, the evidence for the criterion of “time order” was scored as weakly supportive.
Colony loss monitoring does not show a correlation with the use pattern of pesticides (Kluser et al. 2010; vanEngelsdorp et al. 2010, 2011; vanEngelsdorp and Meixner 2010). In Canada, where most honey production is from bees foraging on canola, hive numbers and productivity have increased steadily since neonicotinoid-treated canola seed were introduced (Canadian Honey Council 2009; Statistics Canada 2012). In contrast, high colony losses have occurred on Vancouver Island, where agrochemical use is virtually non-existent (http://www.cbc.ca/news/canada/british-columbia/story/2010/03/09/bc-vancouver-island-bees-die.html). Neonicotinoids are used in Australia, where honey bee losses have not occurred. Conversely, imidacloprid use was restricted in France beginning in 1999, yet reduced survival rates of adult honey bees have continued to be observed since that time. Chauzat et al. (2010) investigated colony mortality in the winter of 2005–2006 in 20 French apiaries, finding no significant residues of agricultural pesticides. The criterion of co-occurrence is thus scored “−.”
A cause–response relationship has been demonstrated for neonicotinoids in laboratory studies and contact and oral LD50 values are known (Hopwood et al. 2012; Blacquière et al. 2012) but the nature of effects at field-realistic doses is debatable. Henry et al. (2012) reported that sub-lethal concentrations of thiamethoxam reduced homing behavior, but the exposure levels were considered unrealistically high (EFSA 2012; DEFRA 2013). Cresswell (2011) performed a meta-analysis of 14 published studies on the effects of imidacloprid on honey bees under laboratory and semi-field conditions, reporting that fitted dose-response relationships estimate that trace dietary levels at field-realistic levels will have no lethal effects but will cause sublethal effects expressed as 6–20% reduced performance. This conclusion contrasts with that of Cutler and Scott-Dupree (2007), who found no adverse chronic effects in field trials in which honey bee colonies were exposed to clothianidin-treated seeds of canola. Studies have shown no adverse acute effects on bees from imidacloprid-treated seeds of maize (Nguyen et al. 2009). Laboratory evidence for a cause–response relationship with neonicotinoids was judged equivocal by the workshop experts, while the evidence from field studies was scored as “somewhat weakening” the support for this cause, although one expert noted that field studies currently being conducted appear to support a cause–response relationship. No link has been found between the expression of genes that code for proteins associated with detoxification of insecticides and collapsed colonies (Johnson et al. 2009).
The mechanism of action of neonicotinoids is known. They are neurotoxins, acting as agonists of insect acetylcholine receptors, disrupting the nervous system and causing death (Matsuda et al. 2001). While the doses needed to produce mortality are well understood, fewer studies have assessed effects at low doses. The evidence for this causal criterion was considered “somewhat supportive.” However, the symptoms resulting from exposure to neonicotinoids are not particularly specific, and not all are directly lethal. Schneider et al. (2012) reported that neonicotinoids can cause behavioral changes (e.g., proboscis extention reflex), reduce foraging activity, and increase foraging flight distance. Decourtye et al. (2003) reported that imidacloprid can affect the learning performance of honey bees. Some of these same symptoms have also been reported as effects of Varroa plus viruses, as discussed previously.
There are some studies that suggest an interaction between pesticides, including neonicotinoids, and other of the candidate causes as potential contributors to colony loss. The intentional use of antibiotics and miticides may enhance bees' susceptibility to neonicotinoids (Hawthorne and Dively 2011). Some pesticides (though not neonicotinoids) are known to kill cells in the midgut of immature bees at sublethal doses, raising the concern that pesticides may affect disease resistance and nutrition absorption (Gregorc and Ellis 2011; Ellis 2012). Interactions between neonicotinoids and Nosema have been reported (Alaux et al. 2010; Pettis et al. 2012; Vidau et al. 2011), although results are inconsistent. For example Pettis et al. (2012) reported increased Nosema infection levels in bees exposed to imidacloprid while Alaux et al. (2010) reported just the opposite. Thompson (2012) summarized evidence from recent monitoring studies in Europe that assessed both pesticide residues within colonies and the presence of pests and pathogens; none clearly identified an interaction between pesticides and disease. The workshop participants concluded that, while neonicotinoids alone were an unlikely cause of honey bee colony losses, they could possibly be a contributing factor.
CONCLUSIONS
A causal analysis methodology was introduced to bee experts at a workshop, in which a problem was defined within a temporal and spatial scale. Experts used a set of causal criteria against which evidence was logically evaluated. Thirty-nine candidate causes were put forth as potential causes of reduced survivability since 2006 of commercial bees used in the California almond industry. Based on the weight-of-evidence against each criterion, candidate causes were categorized as being probable, possible, or unlikely. Due to lack of information and data, several candidate causes were categorized as indeterminate. The parasitic mite Varroa destructor plus viruses was judged to be a “probable cause,” while nutrient deficiency was judged to be a “possible cause” of the reduced survival probability of commercial honey bee colonies in the California almond scenario since approximately 2006. Neonicotinoid pesticides were judged to be “unlikely” as the sole cause of this reduced survival probability, although they could possibly be a contributing factor. The duration of the workshop was insufficient to complete the analysis for all the identified candidate causes (Table 1).
The causal analysis presented here represents a general agreement among the workshop experts, but it is not a formal consensus, and dissenting opinions were expressed on a variety of topics. The results of this analysis should be considered preliminary, because many data gaps remain, especially surrounding environmental factors and beekeeping practices that may potentially affect colony survival but for which the consistency of evidence is “indeterminate” (Table 2). Moreover, it is likely that multiple causes, rather than one single cause, are responsible for the reduced survival of commercial honey bee colonies. The number of potential causes and the likelihood that they are interacting complicates the design of future research investigations. However, a better understanding of these causes can lead to the development of methods to better manage honey bees in commercial beekeeping operations.
ACKNOWLEDGMENTS
We acknowledge the bee experts who attended the workshop: Troy Anderson, Tjeerd Blacquière, Jerry Bromenshenk, Diana Cox-Foster, James Cresswell, Chris Cutler, Galen Dively, Frank Drummond, David Epstein, Richard Fell, Gerald Hayes, Josephine Johnson, Christian Maus, Richard Rogers, Melinda Rostal, Cynthia Scott-Dupree, Thomas Steeger, Helen Thompson, and Geoffrey Williams. Special thanks to Glenn Suter, Susan Cormier, and Erica Fleishman for their expertise in causal analysis. Financial support for this workshop was provided by Bayer CropScience.
Footnotes
Annual is defined as the middle of almond pollination to the middle of this event the following year.
Productive colonies have all stages of brood, good queen, good food stores, and so on.
REFERENCES
- Agricultural Research Council (ARC LNR) Sacbrood. 2010. Available at: http://www.arc.agric.za/home.asp?PID=3062&ToolID=63&ItemID=3082 (accessed August 20, 2012)
- Alaux C, Brunet J-L, Dussaubat C, et al. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera) Environ Microbiol. 2010;12:774–82. doi: 10.1111/j.1462-2920.2009.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux CF, Ducloz F, Crauser D, Le Conte Y. Diet effects on honeybee immunocompetence. Biology Letters. 2010;6:562–7. doi: 10.1098/rsbl.2009.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M, Ball BV. The incidence and world distribution of the honey bee viruses. Bee World. 1996;77:141–62. [Google Scholar]
- Amdam G, Hartfelder K, Norberg K, et al. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): A factor in colony loss during overwintering. J Econ Entomol. 2004;97:741–7. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- Aronstein KA, Murray KD. Chalkbrood disease in honey bees. Journal of Invertebrate Pathology. 2010;103:520–9. doi: 10.1016/j.jip.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ball BV, Allen MF. The prevalence of pathogens in honey bee colonies infected with the parasitic mite Varroa jacobsoni. Ann Appl Biol. 1988;113:237–44. [Google Scholar]
- Beeologics. Israeli acute paralysis virus. Available at: http://www.beeologics.com/iapv.asp (accessed August 22, 2012)
- Biosecurity Authority Ministry of Agriculture and Forestry. 2003. Available at: http://www.biosecurity.govt.nz/files/regs/imports/risk/honey-bee-genetic-material-ra.pdf (accessed August 24, 2012)
- Blacquière T, Smagghe G, van Gestel CAM, et al. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicol. 2012;21:973–92. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Walker PL, Gunn A. The effect of the ectoparasitic mite, Varroa destructor, on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate and lipid levels. Entomol Experi Appl. 2001;101:207–17. [Google Scholar]
- British Columbia Ministry of Agriculture. Kashimir Bee Virus. 2012. Apiculture Factsheet #230, Available at: http://www.agf.gov.bc.ca/apiculture/factsheets/230_kashmir.htm (accessed August 8, 2012)
- Brodschneider R, Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010;41:278–84. [Google Scholar]
- Bromenshenk JJ, Henderson CB, Wick CH, et al. Iridovirus and Microsporidian linked to honey bee colony decline. PLoS ONE. 2010;5(10):e13181. doi: 10.1371/journal.pone.0013181. doi:10.1371/journal.pone.0013181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley LM. The effects of miticides on the reproductive physiology of honey bee (Apis mellifera L.) queens and drones. 2007. Thesis submitted to the faculty of Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science.
- Calderone N. Management of honey bee brood diseases. Part I: Identification and treatment, Dyce Laboratory for Honey Bee Studies. Ithaca, NY: Department of Entomology, Cornell University; 2001. Available at http://www.masterbeekeeper.org/B_files/disease1.htm (accessed March 12, 2012) [Google Scholar]
- Canadian Association of Professional Apiculturists. undated, “Nosema disease – diagnosis and control.” Available at http://www.capabees.com/main/files/downloads/nosema.pdf (accessed February 22, 2012)
- Canadian Honey Council. Pollinating Hybrid Canola—The Southern Alberta Experience. Calgary AB, Canada. HiveLights: H. Clay, Chief Executive Officer, Canadian Honey Council; 2009. pp. 14–6. [Google Scholar]
- Carman H. ARE Update. 5. Vol. 14. University of California, Giannini Foundation of Agricultural Economics; 2011. The Estimated Impact of Bee Colony Collapse Disorder on Almond Pollination Fees; pp. 9–11. Available at http://giannini.ucop.edu/media/are-update/files/articles/v14n5_4.pdf (accessed February 14, 2012) [Google Scholar]
- Chauzat M-P, Martel A-C, Zeggane S, et al. A case control study and a survey on mortalities of honey bee colonies (Apis mellifera) in France during the winter of 2005–6. J Agric Res. 2010;49:40–51. [Google Scholar]
- Cobey SW, Sheppard WS, Tarpy DR. Status of breeding practices and genetic diversity in U.S. honey bees. In: Sammataro D, Yoder JA, editors. Honey Bee Colony Health: Challenges and Sustainable Solutions. Boca Raton, FL, USA: CRC Press; 2012. pp. 25–36. [Google Scholar]
- Core A, Runckel C, Ivers J, et al. A new threat to honey bees, the parasitic phorid fly Apocephalus borealis. PloS ONE. 2012;7(1):e29639. doi: 10.1371/journal.pone.0029639. Available online at: http://www.plosone.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier SM, Suter GW, Norton SB. Causal characteristics for ecoepidemiology. Hum Ecol Risk Assess. 2010;16:53–73. [Google Scholar]
- Cornman RS, Tarpy DR, Chen Y, et al. Pathogen webs in collapsing honey bee colonies. PLoS ONE. 2012;7(8):e43562. doi: 10.1371/journal.pone.0043562. Doi:10.1371/journal.pone.0043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster DL, Conlan S, Holmes EC, et al. A metagenomics survey of microbes in honey bee colony collapse disorder. Science. 2007;318:283–97. doi: 10.1126/science.1146498. [DOI] [PubMed] [Google Scholar]
- Cresswell JE. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicol. 2011;20:149–57. doi: 10.1007/s10646-010-0566-0. [DOI] [PubMed] [Google Scholar]
- Cresswell JE, Desneux N, vanEngelsdorp D. Dietary traces of neonicotinoid pesticides as a cause of population declines in honey bees: An evaluation by Hill's epidemiological criteria. Pest Manage Sci. 2012;68:819–27. doi: 10.1002/ps.3290. [DOI] [PubMed] [Google Scholar]
- Cutler GC, Scott-Dupree CD. Exposure to clothianidin seed-treated canola has no long-term impact on honey bees. J Econ Entomol. 2007;100:765–72. doi: 10.1603/0022-0493(2007)100[765:etcsch]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Decourtye A, Lacassie E, Pham-Deleque M-H. Learning performances of honeybees (Apis mellifera L.) are differentially affected by imidacloprid according to the season. Pest Manage Sci. 2003;69:269–78. doi: 10.1002/ps.631. [DOI] [PubMed] [Google Scholar]
- DEFRA (Department for Environment, Food and Rural Affairs) An Assessment of Key Evidence about Neonicotinoids and Bees. PB 13937. 2013. Available at https://www.gov.uk/government/publications/an-assessment-of-key-evidence-about-neonicotinoids-and-bees.
- De Grandi-Hoffman G, Chen Y, Simonds R. The effects of pesticides on queen rearing and virus titers in honey bees (Apis mellifera L.) Insects. 2013;4:71–89. doi: 10.3390/insects4010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong D. Mites: Varroa and other parasites of brood. In: Morse RA, Flottum K, editors. Honey Bee Pests, Predators and Diseases. Medina, OH, USA: A.I. Root Company; 1997. pp. 200–18. [Google Scholar]
- De Miranda JR, Gautheir L, Ribière M. Honey bee viruses and their effect on bee and colony health. In: Sammataro D, Yoder JA, et al., editors. Honey Bee Colony Health: Challenges and Sustainable Solutions. Boca Raton, FL, USA: CRC Press; 2012. pp. 71–102. [Google Scholar]
- Duay P, DeJong D, Engels W. Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie. 2003;34:61–5. [Google Scholar]
- EFSA (European Food Safety Authority) Inventory of EFSA's Activities on Bees. 2012. Supporting Publication 2012-EN-358. Available at http://www.efsa.europa.eu/en/search/doc/358e.pdf. Accessed 20 May 2013.
- Eischen FA. Overwintering performance of honey bee colonies heavily infested with Acarapis woodi (Rennie) Apidologie. 1987;18:293–304. [Google Scholar]
- Eischen FA, Graham RH. Feeding overwintering honey bee colonies infected with Nosema ceranae. In: Proceedings of the American Bee Research Conference. Am Bee J. 2008;148:555. [as cited in Huang 2011] [Google Scholar]
- Ellis J. The honey bee crisis. Outlooks on Pest Management. 2012 Feb. pp. 35–40.
- Ellis JD. Small hive beetle (Aethina tumida) contributions to colony losses. In: Sammataro D, Yoder JA, editors. Honey Bee Colony Health: Challenges and Sustainable Solutions, Ch. 13. Boca Raton, FL, USA: CRC Press; 2012. [Google Scholar]
- Ellis JD, Ellis A. Featured creatures: Small hive beetle. University of Florida IFAS; 2010. Publication No. EENY-474. [Google Scholar]
- Ellis JD, Zettle Nalen CM. Featured creatures: Varroa mite. University of Florida IFAS; 2010. Publication No. EENY-473. Available at http://entnemdept.ufl.edu/creatures/misc/bees/varroa_mite.htm (accessed March 16, 2012) [Google Scholar]
- Ellis JD, Zettle Nalen CM. Featured Creatures: Varroa Mite. University of Florida IFAS; 2010. Publication EENY-473. Available at http://entnemdept.ufl.edu/creatures/misc/bees/varroa_mite.htm. (accessed March 16, 2012) [Google Scholar]
- EMBL-EBI. Acute bee paralysis virus causes paralysis in bees (Apis mellifera). Available at: http://www.ebi.ac.uk/2can/genomes/viruses/Acute_bee_paralysis_virus.html (accessed August 23, 2012)
- Evans JD, Schwarz RS. Bees brought to their knees: Microbes affecting honey bee health Trends in Microbiology. 2011;19(12):614–20. doi: 10.1016/j.tim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- FERA (Food and Environment Research Agency) Foul brood disease of honey bees and other common brood disorders. UK: Published by DEFRA; 2009. [Google Scholar]
- Ferrari TE, Cobb AB. Could sunspots cause colony collapse? 2010. Available at: http://www.growingproduce.com/article/19535/could-sunspots-cause-colony-collapse (accessed March 16, 2013)
- Fries I. Nosema ceranae in European honey bees (Apis mellifera) Journal of Invertebrate Pathology. 2010;103:573–9. doi: 10.1016/j.jip.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Frost E. Effects of a miticide on honebee memory: Is the cure worse than the disease? Orlando, FL: American Bee Research Conference; 2010. January 15, 2010. [Google Scholar]
- Gard NW, Menzie CA. A causal/risk analysis framework for informing endangered species jeopardy reviews for pesticides. Pesticide Regulation and the Endangered Species Act. 2012. ACS Symposium Series 1111. Available at: http://pubs.acs.org/isbn/9780841227033. (accessed May 20, 2013)
- Garedew A, Schmolz E, Lamprecht I. The energy and nutritional demand of the parasitic life of the mite Varroa destructor. Apidologie. 2004;35:419–30. [Google Scholar]
- Gregorc A, Ellis JD. Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pestic Biochem Physiol. 2011;99:200–7. [Google Scholar]
- Haarmann TM, Spivak D, Weaver B, Glenn T. Effects of fluvalinate and coumaphos on queen honey bees (Hymenoptera: Apidae) in two commercial queen rearing operations. Journal of Economic Entomology. 2002;95:28–35. doi: 10.1603/0022-0493-95.1.28. [DOI] [PubMed] [Google Scholar]
- Hathaway B. Study suggests antibiotics might be another suspect in honey bee die-offs. Yale News; 2012. Available at: http://news.yale.edu/2012/10/30/study-suggests-antibiotics-might-beanother-suspect-honey-bee-die (accessed March 16, 2013) [Google Scholar]
- Hawthorne DJ, Dively GP. Killing them with kindness? In-hive medications may inhibit xenobiotic efflux transporters and endanger honey bees. PLosONE. 2011;6(11):1–9. doi: 10.1371/journal.pone.0026796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M, Beguin M, Requier F, et al. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336(6079):348–50. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Herbert EW, Jr, Shimanuki H. Chemical composition and nutritive value of bee-collected and bee stored pollen. Apidologie. 1978;9:33–40. [Google Scholar]
- Highfield AC, Nagar AE, Mackinder LCM, Noel LJ, Hall MJ, Martin SJ, Schroeder DC. Deformed wing virus implicated in overwintering honeybee colony losses. Applied Environmental Microbiology. 2009;75(22):7212–20. doi: 10.1128/AEM.02227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: Association or causation. Proc Royal Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Hopwood J, Vaughan M, Shepherd M, et al. Are Neonicotinoids Killing Bees? A Review of Research into the Effects of Neonicotinoid Insecticides on Bees, with Recommendations for Action. Portland, OR, USA: The Xerces Society for Invertebrate Conservation; 2012. [Google Scholar]
- Huang Z. Honey bee nutrition. Michigan State University; 2011. Available at http://www.beeccdcap.uga.edu/documents/CAPArticle10.html. (accessed March 12, 2012) [Google Scholar]
- Hung AC, Shimanuki H, Portland OR, et al. Bee parasitic mite syndrome: II. The role of Varroa mite and viruses. Am Bee J. 1995;135:702. [Google Scholar]
- Hung AC, Shimanuki H, Portland OR, et al. The role of viruses in bee parasitic mite syndrome: II. The role of Varroa mite and viruses. Am Bee J. 1996;136:731–2. [Google Scholar]
- ISSG (Invasive Species Specialist Group) Ecology of Varroa destructor. 2006. Compiled by National Biological Information Infrastructure (NBII) and IUCN/SSC. Available at http://www.issg.org/database/species/ecology.asp?si=478&fr=1&sts=sss&lang=EN. (accessed March 16, 2012)
- Johnson R. Honey bee colony collapse disorder. 2010. CRS Report for Congress, 7–5700.
- Johnson RM, Evans JD, Robinson GE, et al. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera) Proc Natl Acad Sci. 2009;106:14790–95. doi: 10.1073/pnas.0906970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Peters L, Siegfriedd B, Elis MD. Drug interactions between in-hive miticides and fungicides in honey bees. 2010. Available at: http://www.extension.org/pages/30365/abrc2010-drug-interactions-between-in-hive-miticidesand-fungicides-in-honey-bees (accessed March 16, 2013)
- Kluser S, Neumann P, Chauzat M-P, et al. UNEP Emerging Issues: Global Honey Bee Colony Disorder and Other Threats to Insect Pollinators. Nairobi, Kenya: United Nations Environment Program; 2010. [Google Scholar]
- LeConte Y, Brunet J-L, McDonnell C, Dussaubat C, Alaux C. Interactions between risk factors in honey bees. In: Sammataro D, Yoder JA, editors. Honey Bee Colony Health: Challenges and Sustainable Solutions. Boca Raton, FL, USA: CRC Press; 2012. pp. 215–22. [Google Scholar]
- Liu S, Walshe T, Long G, et al. Evaluation of potential responses to invasive non-native species with structured decision-making. Conserv Biol. 2012;26:539–46. doi: 10.1111/j.1523-1739.2012.01843.x. [DOI] [PubMed] [Google Scholar]
- Martin TG, Burgman MA, Fidler R, et al. Eliciting expert knowledge in conservation science. Conserv Biol. 2011;26:29–38. doi: 10.1111/j.1523-1739.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Mattila HR, Otis GW. Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn. Ecological Entomology. 2007;32:496–505. [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, et al. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci. 2001;22:573–80. doi: 10.1016/s0165-6147(00)01820-4. [DOI] [PubMed] [Google Scholar]
- Menzie CA, Henning MH, Cura J. A phased approach for assessing combined effects from multiple stressors. Environ Health Perspect. 2007;115:807–16. doi: 10.1289/ehp.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mid-Atlantic Apicultural Research & Extension Consortium. Wax moth. MAAREC Publication 4.5. 2000. February.
- Moore J, Aleksey J, Chandler D, Burroughs N, Evans DJ, Ryabov EV. Recombinants between deformed wing virus and Varroa destructor virus—1 may prevail in Varroa destructorinfested honeybee colonies. Journal of General Virology. 2011;92:156–61. doi: 10.1099/vir.0.025965-0. [DOI] [PubMed] [Google Scholar]
- Moore JL, Runge MC. Combining structured decision making and value-of-information analyses to identify robust management strategies. Conserv Biol. 2012;26:810–20. doi: 10.1111/j.1523-1739.2012.01907.x. [DOI] [PubMed] [Google Scholar]
- Mussen E. Diagnosing and treating Nosema disease. 2011. Available at: http://entomology/ucdavis.edu/faculty/Mussen/beebriefs/Nosema_Disease.pdf (accessed February 22, 2012)
- Naug D. Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol Conserv. 2009;142:2369–72. [Google Scholar]
- NC State University. undated. Effects of genetic diversity on honey bee colony phenotype. Available at: http://www.cals.ncsu.edu/entomology/apiculture/research_projects.html (accessed March 16, 2013)
- Nguyen BK, Saegerman C, Pirard C, et al. Does imidacloprid seed-treated maize have an impact on honey bee mortality? J Econ Entomol. 2009;102:616–23. doi: 10.1603/029.102.0220. [DOI] [PubMed] [Google Scholar]
- Oliver R. Sick bees – Part 2: A model of colony collapse. American Bee Journal. 2010;150(9):865–72. [Google Scholar]
- Oregon State University. Black queen-cell virus. 2008. Available at: http://www.science.oregonstate.edu/bpp/insect_clinic/dieseases/black_queen_cell_virus.htm (accessed August 23, 2012)
- Pernal SF, Currie RW. The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.) Behavioral Ecology and Sociobiology. 2001;51:53–68. [Google Scholar]
- Pettis JS, vanEngelsdorp D, Johnson J, et al. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften. 2012;99:153–8. doi: 10.1007/s00114-011-0881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz P, Aumier P, Ziegelmann B. Biology and control of Varroa destructor. J Invertebr Pathol. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Rose R, Dively GP, Pettis J. Effects of Bt corn pollen on honey bees: Emphasis on protocol development. Apidologie. 2007;38(4):368–77. [Google Scholar]
- Runckel C, Flenniken ML, Engel JC, Ruby JG, Ganem D, Andino R, DeRisi JL. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE. 2011;6(6):e20656. doi: 10.1371/journal.pone.0020656. doi:10.1371/journal.pone.0020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammataro D. Global status of honey bee mites. In: Sammataro D, Yoder JA, editors. Honey Bee Colony Health: Challenges and Sustainable Solutions. Boca Raton, FL, USA: CRC Press; 2012. pp. 37–54. [Google Scholar]
- Sammataro DU, Gerson U, Needham G. Parasitic mites of honey bees: Life history, implications, and impact. Annual Reviews in Entomology. 2000;45:519–48. doi: 10.1146/annurev.ento.45.1.519. [DOI] [PubMed] [Google Scholar]
- Sanford MT. Wax moth control. University of Florida, IFAS Extension; 1995. Document ENY121. [Google Scholar]
- Schneider CW, Tautz J, Grunewald B, et al. RFID tracking of sublethal effects of two neonictinoid insecticides on the foraging behavior of Apis mellifera. PLos ONE. 2012;7(1):1–9. doi: 10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CW, Tautz J, Brunewald B, Fuch S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE. 2012;7(1):e30023. doi: 10.1371/journal.pone.0030023. doi:10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki H, Knox DA. Diagnosis of honey bee diseases. Agriculture Handbook Number 690. USDA ARS; 2000. [Google Scholar]
- Simone-Finstrom M, Spivak M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie. 2010;41(3):295–311. [Google Scholar]
- Statistics Canada. Production and value of honey and maple products. 2012. Available at http://www.statcan.gc.ca/daily-quotidien/121214/dq121214c-eng.htm (accessed February 19, 2013)
- Stindl R, Stindl W., Jr Vanishing honey bees: Is the dying of adult worker bees a consequence of short telomeres and premature aging? Medical Hypotheses. 2010;75(4):387–90. doi: 10.1016/j.mehy.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Suter GW, Norton SB, Cormier SM. The science and philosophy of a method for assessing environmental causes. Hum Ecol Risk Assess. 2010;16:19–34. [Google Scholar]
- Thompson HM. Interaction between Pesticides and Other Factors in Effects on Bees. European Food Safety Authority; 2012. Available at www.efsa.europa.eu/publications. (accessed May 20, 2013) Supporting Publications 2012:EN-340. [Google Scholar]
- Thomson JR, Kimmerer WJ, Brown LR, et al. Bayesian change point analysis of abundance trends for pelagic fishes in the upper San Francisco Estuary. Ecol Appl. 2010;20:1431–48. doi: 10.1890/09-0998.1. [DOI] [PubMed] [Google Scholar]
- Underwood RM, vanEngelsdorp D. Colony collapse disorder: Have we seen this before? The Pennsylvania State University Department of Entomology; 2007. Available at: http://www.beeculture.com/content/ColonyCollapseDisorderPDFs/7%20Colony%20Collapse%20Disorder%20Have%20We%20Seen%20This%20Before%20-%20Robyn%20M.%20Underwood%20and%20Dennis%20vanEngelsdorp.pdf (accessed August 23, 2012) [Google Scholar]
- University of Georgia, College of Agriculture and Environmental Sciences. Honey bee disorders: Bacterial diseases. American Foulbrood; 2011. Available at: http://www.ent.uga.edu/bees/disorders/bacterial.html#afb (accessed March 1, 2012) [Google Scholar]
- USDA. Honey bees and colony collapse disorder. 2012. Available at: http://www.ent.uga.edu/bees/disorders/bacterial.html#afb (accessed March 16, 2013)
- USDA BARC (Beltsville Agricultural Research Center) Honey bee tracheal mite – Acarapis woodi. 2002. Available at http://www.ba.ars.udsa.gov/psi/brl/mite-aw.htm (accessed February 2, 2012)
- USEPA (US Environmental Protection Agency) Stressor Identification Guidance Document. Washington, DC, USA: Office of Water; 2000. EPA/822/B-00/025. [Google Scholar]
- USEPA. Analysis of the Causes of a Decline in the San Joaquin kit Fox Population on the Elk Hills, Naval Petroleum Reserve #1, California. Cincinnati, OH, USA: National Center for Environmental Assessment, Office of Research and Development; 2008. EPA/600/R-08/130. [Google Scholar]
- USEPA. CADDIS, The Causal Analysis/Diagnosis Decision Information System. 2012. Available at http://www.epa.gov/caddis/. (accessed February 15, 2013)
- vanEngelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. 2010;103:580–95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D, Cox Foster D, Frazier M, et al. Preliminary Report: First Revision. Harrisburg, PA, USA: Pennsylvania Department of Agriculture; 2007. Fall-dwindle Disease: Investigations into the Causes of Sudden and Alarming Colony Losses Experienced by Beekeepers in the Fall of 2006; p. 22. [Google Scholar]
- vanEngelsdorp D, Speybroeck N, Evans JD, et al. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J Econ Entomol. 2010;103:1517–23. doi: 10.1603/ec09429. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D, Hayes J, Jr., Underwood RM, et al. A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. J Agric Res. 2011;50(1):1–10. [Google Scholar]
- Vidau C, Diogon M, Aufauvre J, et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE. 2011;6(6):e21550. doi: 10.1371/journal.pone.0021550. doi:10.1371/journal.pone.0021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster TC, Delaplane KS. Mites of the Honey Bee. Hamilton, IL, USA: Dadant and Sons, Inc; 2001. [Google Scholar]
- Wickwire T, Menzie CA. The causal analysis framework: Refining approaches and expanding multidisciplinary applications. Hum Ecol Risk Assess. 2010;16:19–18. [Google Scholar]
- Yang X, Cox-Foster D. Effects of parasitization by Varroa destructor on survivorship and physiological traits of Apis mellifera in correlation with viral incidence and microbial challenge. Parasitol. 2007;134:405–12. doi: 10.1017/S0031182006000710. [DOI] [PubMed] [Google Scholar]
- Zawislak J. Managing small hive beetles. University of Arkansas Division of Agriculture; FSA7075. (undated). [Google Scholar]


