Table 1.
Preparation of compounds 3 by one-pot CEYM–Diels–Alder reaction of substrates 1 (method A).
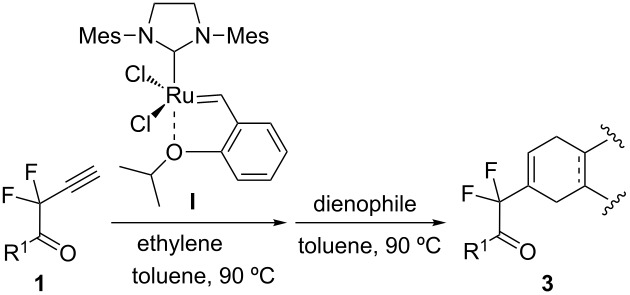 | ||||||
| entry | 1 | R1 | dienophile | product | % yield method Aa (time, h) |
% yield method Bb (time, h) |
| 1 | 1a | Bn–NH | 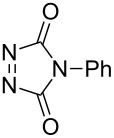 |
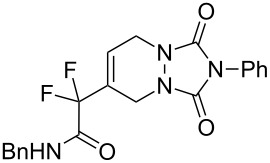 3a |
60 (2 h)c | – |
| 2 | 1a | Bn–NH | 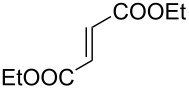 |
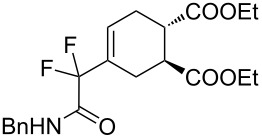 3b |
55 (8 h) | 70 (20 h) |
| 3 | 1a | Bn–NH | 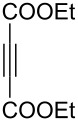 |
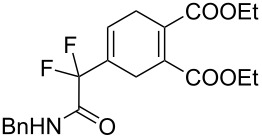 3c |
51 (6 h) | 55 (6 h) |
| 4 | 1a | Bn–NH | 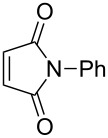 |
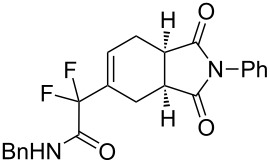 3d |
50 (4 h) | 63 (24 h) |
| 5 | 1a | Bn–NH | 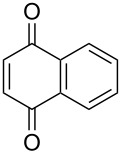 |
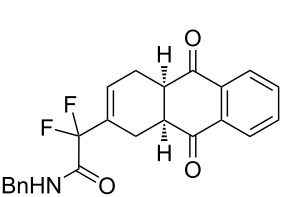 3e |
47 (24 h) | 50 (24 h) |
| 6 | 1a | Bn–NH | 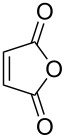 |
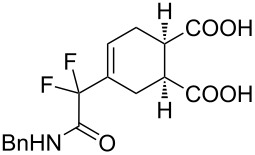 3f |
85 (120 h)c | – |
| 7 | 1b | (R)-Ph(Me)–CHNH | 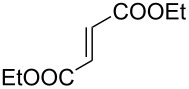 |
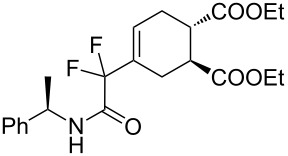 3g + 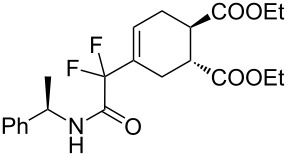 3g’ |
87 (4 h)d | 73 (24 h)d |
| 8 | 1b | (R)-Ph(Me)–CHNH | 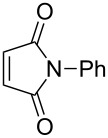 |
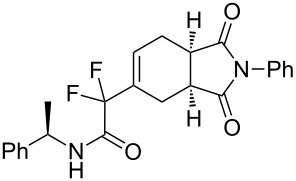 3h + 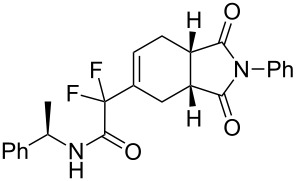 3h’ |
74 (2 h)d | 70 (10 h)d |
| 9 | 1b | (R)-Ph(Me)–CHNH | 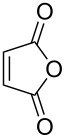 |
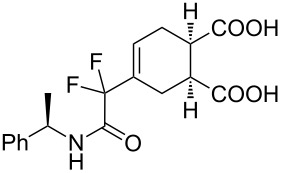 3i + 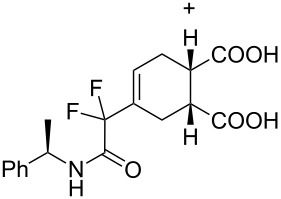 3i’ |
74 (20 h)c,d | – |
| 10 | 1b | (R)-Ph(Me)–CHNH | 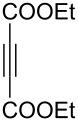 |
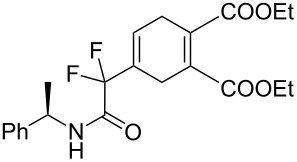 3j |
63 (10 h) | 60 (24 h) |
| 11 | 1c | PropylNH | 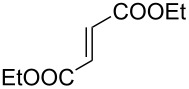 |
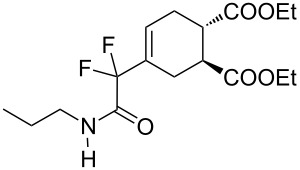 3k |
40 (5 h) | – |
| 12 | 1d | Ph | 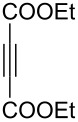 |
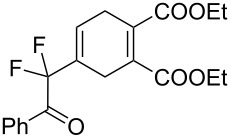 3l |
48 (4 h) | 55 (15 h) |
| 13 | 1d | Ph | 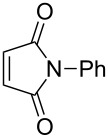 |
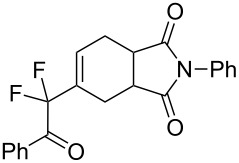 3m |
28 (8 h) | – |
| 14 | 1d | Ph | 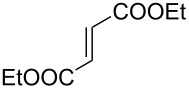 |
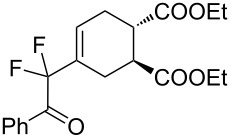 3n |
58 (12 h) | 70 (17 h) |
| 15 | 1e | Cy | 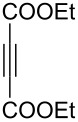 |
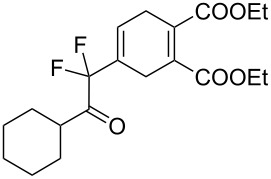 3o |
54 (12 h) | – |
aMethod A: One-pot protocol with Mori´s conditions. The formation of the corresponding diene 2 with ethylene was complete after 2 h at 90 °C for all substrates. bMethod B: Tandem multicomponent protocol mediated by 1,7-octadiene [29]. cWhen maleic anhydride or 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione were used as dienophiles, the Diels–Alder reaction was performed at rt. With maleic anhydride final products were isolated as the corresponding diacid derivatives. dIn all cases, adducts were obtained as an inseparable 1:1 mixture of diastereoisomers.
